1. Background
Escherichia coli is one of the most prevalent gram-negative bacteria that cause several intestinal and other organ infections, such as septicemia, pneumonia, neonatal meningitis, and urinary tract infections (UTIs) (1, 2). Recently, E. coli has become resistant to many antibiotics. In addition to antibiotic resistance, some studies have reported a reduction in the effects of disinfectant compounds on E. coli, which can be an important health problem (3). Reduced effectiveness of biocides may result from consecutive contact of bacteria with biocides (4). Antimicrobial biocides have various effects on microorganisms, such as causing cytoplasmic membrane damage, nucleic acid destruction, or ribosome denaturation (5). In bacteria, the reduction of the effect of biocides can result from intrinsic factors like the outer membrane of gram-negative bacteria, arabinogalactan in the cell wall of mycobacteria, biofilm formation, changes in enzyme structure, expression of stress response genes, efflux pump activity, mutation, or acquisition of genetic elements carrying tolerance genes for biocides, as well as the acquisition or upregulation of efflux pump genes located on plasmids, T3 transposons, composite transposons, or conjugative transposons (6-8).
Hospital-acquired E. coli isolates are resistant to antimicrobial biocides in two ways: The first type of resistance is intrinsic, involving control of the influence of compounds into the cell, and the second involves acquiring an extrinsic gene that reduces the influence of biocides into the cell, reduces permeability, or enhances efflux pump activity (5). mdfA, emrE, qacA/B, qacED1, qacE, qacG, qacH, and qacJ are examples of efflux pump genes found in the plasmid or chromosomal genome of some bacteria, such as Staphylococci and Enterobacteriaceae (9).
Increasing resistance to antimicrobial biocides, along with antibiotics in E. coli isolates, is one of the important public health concerns. Unlike antibiotic resistance studies, we have much less data about the resistance rate to biocides worldwide.
2. Objectives
This study was performed to evaluate the resistance rate of E. coli isolates to common antimicrobial biocides (benzalkonium chloride, chlorhexidine gluconate, triclosan, and formaldehyde) and to detect some important efflux pump-encoding genes responsible for resistance against biocides.
3. Methods
3.1. Bacterial Isolation and Identification
All 200 non-duplicate E. coli isolates were obtained from outpatients with UTIs in Ardabil hospitals from March 2021 to January 2023. All samples were clean-catch midstream urine that was rapidly inoculated in sheep blood agar (Merck, Germany) and eosine methylene blue (EMB) (Kardan Azma, Iran) and incubated at 37℃ for a full day. Every cultured sample with ≥ 105 CFU gram-negative bacilli was inoculated in indole, methyl red, Voges-Proskauer, and citrate (IMVIC) (Merck, Germany) and incubated at 37℃ overnight, and the results were observed. In the present study, 200 isolates of E. coli were collected according to IMVIC results with John G. Holt's standards (10) and stored at -70℃.
3.2. Biocide Susceptibility
To measure the minimum inhibitory concentration (MIC), the microdilution method (11) was performed on all 200 E. coli isolates for formaldehyde, benzalkonium chloride, chlorhexidine digluconate, and triclosan. The tests were conducted in Mueller-Hinton broth, and MIC90 was considered the breakpoint. According to CLSI 2022 recommendations, quality control for this method was done with E. coli ATCC 2592. Briefly, all biocides formaldehyde and triclosan (98%) (Bio Basic, Canada), benzalkonium chloride (> 95%) (Sigma-Aldrich, USA), and chlorhexidine digluconate (20%) (Sigma-Aldrich, USA) in 1 - 128 μg/mL concentrations were added into Mueller-Hinton broth medium (Himedia, India), then added to wells. One μL of a 0.5 McFarland (1.5 × 108 CFU/mL) mixture of fresh isolates was inoculated into each well and incubated at 37℃ for 16 - 20 hours. The lowest concentration of each biocide that inhibits bacterial growth was considered the MIC (11).
3.3. Antimicrobial Biocides Resistance Genes Detection
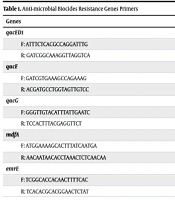
A PCR assay with specific primers (Table 1) was used for the detection of efflux-encoding genes (emrE, mdfA, qacE, qacG, and qacED1) according to a previous study protocol (12). The annealing temperature for each primer is shown in Table 2.
| Genes | Annealing (°C) | Product Size (bp) |
|---|---|---|
| qacED1 | 59 | 323 |
| F: ATTTCTCACGCCAGGATTTG | ||
| R: GATCGGCAAAGGTTAGGTCA | ||
| qacE | 50 | 258 |
| F: GATCGTGAAAGCCAGAAAG | ||
| R: ACGATGCCTGGTAGTTGTCC | ||
| qacG | 56 | 122 |
| F: GGGTTGTACATTTATTGAATC | ||
| R: TCCACTTTACGAGGTTCT | ||
| mdfA | 58 | 513 |
| F: ATGGAAAAGCACTTTATCAATGA | ||
| R: AACAATAACACCTAAACTCTCAACAA | ||
| emrE | 55 | 420 |
| F: TCGGCACCACAACTTTTCAC | ||
| R: TCACACGCACGGAACTCTAT |
| Steps | Temperatures and Times | Cycles |
|---|---|---|
| Initial denaturation | Four min at 94°C | 1 |
| Denaturation | One min at 94°C | 30 |
| Annealing | One min (temperatures are shown for each primer in Table 1 ) | 30 |
| Extension | One min at 72°C | 30 |
| Final extention | One min at 72°C | 1 |
3.4. Data Analysis
This investigation examined the MICs of antimicrobial biocides in E. coli strains carrying resistance genes. Statistical analysis was conducted using SPSS (version 16), with the chi-square test applied to assess potential relationships between efflux pump-encoding genes and biocide MIC values. A statistically significant association (P < 0.05) was observed, suggesting that the presence of these genes correlates with heightened biocide resistance in the bacterial isolates.
3.5. Ethics Statement
Ethical oversight for this investigation was provided by the Ardabil University of Medical Sciences' Ethics Committee (approval code: IR.ARUMS.MEDICINE.REC.1402.001), ensuring compliance with Helsinki Declaration guidelines (1975 version). Participant involvement was contingent upon the completion of written informed consent procedures.
4. Results
Of all 200 E. coli isolates, formaldehyde and benzalkonium chloride, with an MIC90 of 64 μg/mL, showed the highest resistance. In contrast, chlorhexidine digluconate and triclosan, with MIC90 values of 16 and 4 μg/mL, respectively, exhibited lower resistance and high efficiency (Table 3). The prevalence of genes encoding efflux pumps responsible for resistance against antimicrobial biocides showed that mdfA had the highest prevalence at 88.5%. The rates for emrE, qacED1, qacG, and qacE were 80%, 24.5%, 6%, and 4%, respectively. The qacED1 and qacE were responsible for high-level resistance to benzalkonium chloride, chlorhexidine digluconate, and triclosan (P ≤ 0.05). mdfA and emrE were correlated with resistance to formaldehyde, triclosan, and chlorhexidine digluconate, respectively (P ≤ 0.05). Additionally, the presence of qacG is related to resistance against benzalkonium chloride and chlorhexidine digluconate (P ≤ 0.05) (Table 4).
| Anti-microbial Biocides | MIC (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | MIC90 | |
| Formaldehyde | - | - | - | - | 14 (7) | 52 (26) | 114 (57) | 20 (10) | 64 |
| Benzalkonium chloride | - | - | - | - | 97 (48.5) | 76 (38) | 27 (13.5) | - | 64 |
| Chlorhexidine digluconate | - | 48 (24) | 57 (28.5) | 65 (32.5) | 30 (15) | - | - | - | 16 |
| Triclosan | 110 (55) | 38 (19) | 38 (19) | 14 (7) | - | - | - | - | 4 |
Abbreviation: MIC, minimum inhibitory concentration.
a Values are expressed as No. (%).
| Genes | Anti-microbial Biocides (MIC µg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formaldehyde | Benzalkonium Chloride | Chlorhexidine Digluconate | Triclosan | |||||||||
| ≤ 32 | ≥ 64 | P-Value | ≤ 32 | ≥ 64 | P-Value | ≤ 8 | ≥ 16 | P-Value | ≤ 2 | ≥ 4 | P-Value | |
| qacED1 | 0.68 | > 0.01 | > 0.01 | > 0.01 | ||||||||
| qacED1+ | 15 | 34 | 22 | 27 | 19 | 30 | 1 | 48 | ||||
| qacED1- | 51 | 100 | 151 | 0 | 151 | 0 | 147 | 4 | ||||
| qacG | 0.06 | < 0.01 | < 0.01 | 0.62 | ||||||||
| qacG+ | 1 | 11 | 0 | 12 | 0 | 12 | 7 | 5 | ||||
| qacG- | 65 | 123 | 173 | 15 | 170 | 18 | 141 | 47 | ||||
| qacE | 0.62 | > 0.01 | > 0.01 | 0.03 | ||||||||
| qacE+ | 2 | 6 | 0 | 8 | 2 | 6 | 0 | 8 | ||||
| qacE- | 64 | 128 | 173 | 19 | 168 | 24 | 148 | 44 | ||||
| mdfA | > 0.01 | 0.94 | 0.12 | 0. 017 | ||||||||
| mdfA+ | 46 | 131 | 153 | 24 | 148 | 29 | 125 | 52 | ||||
| mdfA- | 20 | 3 | 20 | 3 | 22 | 1 | 23 | 0 | ||||
| emrE | 0.49 | 0.21 | 0.047 | > 0.01 | ||||||||
| emrE+ | 51 | 109 | 136 | 24 | 132 | 28 | 108 | 52 | ||||
| emrE- | 15 | 25 | 37 | 3 | 38 | 2 | 40 | 0 | ||||
Abbreviation: MIC, minimum inhibitory concentration.
5. Discussion
In this study, the highest level of resistance to formaldehyde and benzalkonium chloride was observed at 64 µg/mL. Oosterik et al. in Belgium (13) obtained E. coli isolates with MIC90 levels between 40 and 80 µg/mL, which were similar to our results. In addition, in a recent study conducted by us (14) on Pseudomonas aeruginosa isolates, the MIC90 of formaldehyde was 512 µg/mL, which is a higher level than in this study. This can be attributed to the intrinsic characteristics of P. aeruginosa (14). Also, the level of resistance to chlorhexidine digluconate was lower, with an MIC90 of 16 µg/mL. This result is similar to the study by da Silva in Germany (15) and, compared to the study by Beier et al. on Campylobacter (16), it is at a higher level. Although the sensitivity of all disinfectants has been decreasing over the years, fortunately, based on the systematic study by Buxer, the sensitivity of E. coli isolates to chlorhexidine digluconate has increased over the past 50 years (17).
Triclosan was the most effective disinfectant used in this study. The MIC90 for it was 4 µg/mL, which is similar to the result obtained in Germany (18), although there was another study in Germany that had an MIC level higher than 1000 µg/mL (19). This could be due to increased exposure (20). However, in our study, this biocide was the most effective disinfectant, and therefore it can be used against cases of E. coli isolates that are resistant to other biocides. Triclosan is one of the most effective biocides against bacteria and is widely used in industry and health-related organizations (21, 22). Therefore, identifying triclosan as the most effective biocide against E. coli in Ardabil can be the starting point for similar research on other important microorganisms' resistance patterns against triclosan, so that this disinfectant can be used effectively in relevant organizations. Furthermore, according to Russell’s report about no relation between the usage of triclosan and increasing antibiotic drug resistance (22), we can use triclosan with greater confidence.
Efflux pumps are a key bacterial mechanism for resisting stressors such as biocides and antibiotics, and qac is a common efflux gene. These genes can spread in bacterial populations through the conjugation process and cause resistance against antimicrobial compounds among them (23). The prevalence of mdfA, emrE, qacED1, qacG, and qacE efflux genes was obtained by the PCR method, and the results are 88.5%, 80%, 24.5%, 6%, and 4%, respectively. In this study, like other studies (24-26), we indicate that the presence of efflux genes is related to the level of antimicrobial resistance. We designed this study with the aim of evaluating the resistance level of E. coli isolates to disinfectants and determining the correlation between the presence of efflux pump genes and the level of resistance; we clarified the relationship between these two factors. It would have been better if other bacteria that cause UTIs and are known as resistant bacteria against biocides or antibiotic drugs, such as Acinetobacter, were also included in the study. However, they were not included due to cost constraints.
5.1. Conclusions
The results of this study showed that the level of resistance to antimicrobial biocides in E. coli isolates from UTIs is high and is considered one of the public health threats. If they contaminate surfaces, we cannot eradicate them easily. Although the MIC90 level of E. coli isolates to most of the examined biocides was high, the level of resistance to triclosan was low, making it one of the trusted options for disinfecting surfaces from E. coli. Additionally, based on the results obtained from this study, there is a positive relationship between the presence of efflux pump genes and antimicrobial resistance.