1. Background
Toxoplasma gondii, an intracellular parasite, is classified under the coccidian subclass. The tachyzoites of T. gondii are involved in acute infection since tissue cysts and bradyzoites are responsible for latent infection (1).
Toxoplasma infection in immune-capable adults is mostly benign and without any physical signs. In some cases, it can influence mononucleosis-like symptoms. In chronic infections, T. gondii bradyzoites grow to tissue cysts in the central nervous system and other organs. T. gondii tissue cysts may remain unknown for several years and the host immune system may be threatened by released bradyzoites (2, 3). Although T. gondii has affected nearly one-third of the world’s population, the distribution of toxoplasmosis infection varies in different parts of the world (4). Previous studies showed a prevalence of 18 to 70% in different areas of Iran (5).
Alzheimer’s disease is the main focus of the majority of dementia studies. As Alzheimer’s disease is responsible for 60% of dementia cases, most neuropsychological studies have focused on Alzheimer’s disease. Accurate clinical diagnosis of dementia as well as its differentiation from the normal aging process have a significant effect on the diagnosis of the disease and the early use of specific treatment. By considering this fact and the absence of a complete diagnostic index for identifying these patients, looking for a good diagnostic approach and screening in these patients could be important (6).
The association of T. gondii infection with neuropsychiatric disorders such as Parkinson’s disease (PD), schizophrenia, depression, bipolar and anxiety disorders has been the goal of several projects (7-11). Evaluations revealed that T. gondii infection could influence human and animal behaviors (12). The occurrence of T. gondii infection was reported about three times further in schizophrenic people (13).
It has been suggested that neurotransmitter dysregulation is the main mechanism underlying the induction of schizophrenia by this parasite (8, 11).
Dementia is a common disorder that has affected 5% of the population over 65-years-old and 30% of the population over 80-years-old. This malfunction has been characterized by the loss of memory, impairment in daily activities, communication, judgment, and ability to focus. Alzheimer’s disease comprises 60% to 80% of dementia cases. Vascular dementia, brain stroke, thyroid problems, and vitamin deficiencies are other probable causes of dementia (6).
2. Objectives
According to the etiological relationship between T. gondii infection and neurodegenerative disorders, a few studies have been conducted to evaluate the relationship between toxoplasmosis and dementia. The present study tried to evaluate the prevalence of T. gondii infection among individuals with dementia in comparison with healthy controls without a history of neurodegenerative disease.
3. Methods
3.1. Study Population
Based on clinical manifestations, we selected a sample of 100 patients with dementia and 99 healthy volunteers from urban districts referring to hospitals of Arak and Hamadan University of Medical Sciences, Iran. The groups were matched for gender, age, and socioeconomic status. The inclusion criteria were the positive diagnosis of dementia and written informed consent. The mental status of both groups was examined by a neurologist via Addenbrooke’s cognitive examination (ACE-R) (14). Healthy control individuals were screened to rule out any psychiatric disease. After signing the consent forms, demographic data such as age, gender, dietary habits, and contact with cats were gathered through pre-designed questionnaires. The study protocol was submitted, reviewed, and approved by the Ethics Committee of the Hamadan University of Medical Sciences (ethical code: IR.UMSHA.REC.1396.299)
3.2. Serological Test
We obtained a 2 mL blood sample from each individual and centrifuged at 900 g for 10 minutes. The samples were divided and kept at -20°C until use. All sera were assessed for T. gondii IgG and IgM antibodies by enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (Pishtazteb Co., Iran) following the manufacturer’s instructions. Based on the instruction, the values of higher than 1.1 IU/mL were considered positive and lower than 0.9 IU/mL were considered negative. The values between 0.9 and 1.1 were observed as suspicious and tested again.
3.3. Statistical Analysis
Statistical analysis was performed by SPSS version 22 software. The proportions were calculated with a confidence interval of 95% using the chi-square and Fisher’s exact tests at a significance level of 5%.
4. Results
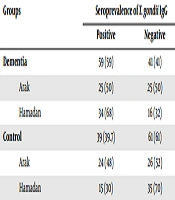
A total of 199 individuals (100 dementia patients and 99 healthy controls) were examined in the present study. We found that 59 (59%) dementia patients were infected with T. gondii. Moreover, 39 (39.3%) control subjects were positive for anti-T. gondii IgG antibodies. Significant differences were observed in the level of IgG antibody between dementia and control groups (P = 0.002). Two dementia patients were reported to be positive for T. gondii IgM antibodies (prevalence, 2%). No positive case of the T. gondii IgM antibody was observed in the control group (Table 1). The seroprevalence of toxoplasmosis was higher in dementia patients from Hamadan than patients from Arak (68% versus 50%, with no significant difference). The average age of participants was 77.06 ± 6.7 years. There were 114 males (57%) and 86 females (43%) in the sample. A significant relationship was found between marital status and dementia (P = 0.021). There was evidence of statistically significant association between latent toxoplasmosis and variables such as contact with cat (P < 0.001) and meat consumption (cooked or raw) in the diet (P = 0.015). The detail characteristics of dementia patients and control subjects are shown in Table 2, including age distribution, marital status, education, contact with cats, and the way of food consumption and water drinking.
| Groups | Seroprevalence of T. gondii IgG | |
|---|---|---|
| Positive | Negative | |
| Dementia | 59 (59) | 41 (41) |
| Arak | 25 (50) | 25 (50) |
| Hamadan | 34 (68) | 16 (32) |
| Control | 39 (39.7) | 61 (61) |
| Arak | 24 (48) | 26 (52) |
| Hamadan | 15 (30) | 35 (70) |
aValues are expressed as No. (%).
| Characteristics | IgG Seropositivity in Case Group | IgG Seropositivity in Control Group |
|---|---|---|
| Gender | ||
| Male | 62 (31) | 52 (26) |
| Female | 38 (19) | 48 (24) |
| Marital status | ||
| Married | 74 (40.4) | 92 (50.2) |
| Single | 9 (4.9) | 8 (4.3) |
| Education | ||
| Illiterate | 37 (20.3) | 40 (21.9) |
| Elementary school | 18 (9.8) | 14 (7.69) |
| Secondary school | 17 (9.34) | 18 (9.8) |
| High school | 15 (8.24) | 26 (14.28) |
| Academic degree | 1 (0.54) | 2 (1.09) |
| Cat exposure | ||
| Having companion cats | 4 (2.19) | 8 (4.39) |
| Having cats around | 66 (36.2) | 70 (38.46) |
| Having no cats around | 9 (4.94) | 22 (12.08) |
| Meat consumption | ||
| Totally cooked | 52 (28.4) | 54 (29.5) |
| Raw or semi-cooked | 23 (12.5) | 31 (16.9) |
| No meat in the diet | 8 (4.3) | 15 (8.1) |
| Vegetables washing | ||
| Using disinfectants | 28 (15.3) | 31 (17) |
| Using salt or vinegar | 21 (11.5) | 3 (18.6) |
| No disinfection | 10 (5.49) | 0 (0) |
| Milk consumption | ||
| Industrial pasteurized milk | 37 (20.3) | 39 (21.4) |
| Traditional milk (boiled) | 43 (23.6) | 60 (32.9) |
| Raw milk | 3 (1.6) | 1 (0.54) |
| Egg consumption | ||
| Hard-boiled egg | 43 (23.4) | 64 (34.9) |
| Soft-boiled egg | 39 (21.3) | 36 (19.6) |
| Raw egg | 1 (0.5) | 0 (0) |
| Water drinking | ||
| Purified water | 26 (14.2) | 45 (24.5) |
| Tap water | 55 (30) | 55 (30) |
| Spring water | 2 (1) | 0 (0) |
aValues are expressed as No. (%).
5. Discussion
Evaluations showed that the CNS is the most affected organ for T. gondii infection. This parasite can enter CNS cells such as glia and neurons to dysregulate neurotransmitters (11, 15-18). The current study was conducted to compare the seroprevalence of toxoplasmosis in dementia and healthy control groups in Arak and Hamadan provinces, West of Iran. In the present study, the prevalence of Toxoplasma-specific IgG antibody was significantly higher in the serum of dementia patients than in controls (59% versus 39.3%). The study showed two positive cases of IgM antibody against toxoplasmosis in dementia patients. There are numerous studies on the role of T. gondii in neurological diseases. Menati Rashno et al. reported that the overall prevalence of T. gondii infection was 66% and 56.3% in patients with AD and controls, respectively (19). In agreement with the present study, the toxoplasmosis prevalence was higher in the test group than in the healthy group. In a study by Bouscaren et al. in central Africa, no statistically significant relationship was reported between toxoplasmosis seropositivity and dementia (20). The possible reason between the mentioned study and our result may be related to this fact that dementia was not perused as a probable risk factor of dementia. Kusbeci et al. described that 44.1% of Alzheimer’s disease cases and 24.3% of healthy controls were positive for anti-IgG antibodies. They also indicated a statistically significant difference between the rates of positivity between AD patients and controls (P = 0.005) (9). The higher seroprevalence of toxoplasmosis in individuals with dementia than in the control group showed the possible effect of this parasite on the occurrence of dementia symptoms in the present study and previous studies. Therefore, toxoplasmosis can be a possible cause of dementia that requires special attention of neurological specialists.
In a study by Geschwind, the presence of toxoplasmosis was confirmed in the brain autopsy of a 28-year-old patient with progressive dementia (21). In different studies, dementia has been associated with some organisms that can infect the CNS. For instance, in 2016, Le and Spudich showed dementia in HIV patients who did not take any medications (22). Also, Ances and Ellis in 2007 showed that brain dementia was a common complication seen in 50% of HIV patients without any treatment (23). Other neurodegenerative diseases have been investigated in different studies. For example, Mahami Oskouei et al. implied no significant correlation between PD and toxoplasmosis (5). In this study, 85% of PD patients and 90.3% of controls were positive for anti-Toxoplasma IgG antibody. The investigation of the seroprevalence of toxoplasmosis in a case-control study by Fallahi et al. showed 53% IgG titer in PD patients and 55.6% in controls, showing no statistically significant association between Toxoplasma seropositivity and PD (24).
There was a significant correlation between the prevalence of toxoplasmosis and the consumption of red meat so that 100% of people from Hamadan and 90% of the same group from Arak who did not have an interest in meat consumption had no history of toxoplasmosis (P < 0.05). This finding suggested that consuming meat in any form (cooked or raw) can increase the risk of toxoplasmosis and this is especially important in high-risk groups. Menati Rashno et al. found similar results that the protein content was higher in the diet of people who had positive IgG titers against toxoplasmosis than in the diet of people who were using more vegetables (19).
We also found that the level of antibody was lower in people who kept cats or were not in contact with cats than in those who were in contact with street cats. This could be a warning that street cats are infected and we need to prevent the contact of street cats with sensitive people, including pregnant women, children, and the elderly. In a study by James et al. the seroprevalence of toxoplasmosis was higher in people who were in contact with street cats; however, this difference was not significant (25).
According to the results of the serological assay, the Toxoplasma-specific IgM antibody was found in two patients with dementia. The absence of a significant number of acute toxoplasmosis in the test group was expected. In other studies that examined the association between toxoplasmosis and other neurodegenerative diseases such as schizophrenia, the absence of acute cases of toxoplasmosis was recorded (26).
Overall, the results of this study showed that a higher percentage of patients with dementia are infected with toxoplasmosis. Extensive studies with higher sample sizes are needed to explain the relationship between dementia and toxoplasmosis. As long as the link between toxoplasmosis and dementia is clearly identified, the emphasis on preventive measures needs to be taken into account in preventing Toxoplasma gondii infection and its chronicity.