1. Background
Degenerative joint disease (DJD) is considered the leading cause of disability in older adults. About 4% of the general population is involved with a condition that is responsible for a huge burden on the global health system (1, 2). Despite standard surgical treatments such as arthroplasty, hemi-arthroplasty, and osteotomy (3), treatments with consistent evidence include land and water-based exercises, endurance exercises (quadriceps muscle strengthening), and non-steroidal anti-inflammatory drug (NSAID) administration (4-7). The purpose of treatment is to relieve pain, improve function and maintain joint mobility (8). Oral NSAID administration, as maintenance treatment, tends to reduce pain and symptoms rather than providing a "cure". Since NSAIDs have adverse gastrointestinal effects, they cannot be used for more than a specific duration (9).
Consequently, the intra-articular injection methods were proposed to postpone the degenerative processes or even trigger the healing and anabolic pathways. Intra-articular injections are mini-invasive methods, the most important of which are injections of hyaluronic acid (HA) and platelet-rich plasma (PRP). Controversial results were seen for both PRP and HA with different injection protocols in different studies. Albeit, after a recent meta-analysis by Rutjes et al. (10), several studies have tended slightly toward PRP (11).
In the study of Patel et al., 78 patients (156 knees) with osteoarthritis were injected with PRP (52 knees with a single PRP injection; 50 knees with two injections of PRP 3 weeks apart) or placebo (46 knees with normal saline) without any further conservative treatment. It was found that PRP provided a significantly better functional response compared to placebo in 6 months follow-up (12).
2. Objectives
In the present study, we also compared the therapeutic effects of PRP injection with normal saline injections (control group). We also evaluated the effects of underlying factors such as age, gender, and body mass index (BMI) on the therapeutic effects of PRP to see if any of these factors could have a significant effect on the therapeutic outcomes. In order to ensure that the control group had minimal treatment during the follow-up period, we also used a conservative treatment in both groups. Our hypothesis was that the PRP group would have a better result than the control group.
3. Methods
3.1. Study Design
In this single-blind randomized clinical trial, patients who suffered from moderate-grade knee osteoarthritis (grade 2 based on radiological Kellgren-Lawrence classification) were enrolled. The study was also registered in the Iranian Registry of Clinical Trials (No. IRCT201301167274N6), and the study was conducted in accordance with the Helsinki Declaration. The pros and cons of both methods were explained to the patients, and informed consent was obtained.
3.2. Inclusion and Exclusion Criteria
The inclusion criteria were the existence of chronic knee pain for more than six months, age of 40 - 65 years, confirmed grade 2 at radiological signs in Kellgren-Lawrence classification by the senior author, no history of intra-articular injection during the past three months, no history of knee surgery and/or severe knee trauma or deformity, and no pregnancy. Oral NSAID consumption during the last week before enrollment, arbitrary use of other NSAIDs during the study, history of hematologic problems (coagulopathy) or anticoagulant drugs, history of severe cardiovascular disease, hemoglobin levels < 11 g/dL or platelet count < 150,000 per mL of blood were excluded.
Upon injecting PRP or saline, a “conservative treatment” was administered for both groups for two reasons. First, patients may self-treat with analgesic medications if they do not respond to treatment. Therefore, this conservative treatment would diminish the likelihood of this bias. Second, this is contrary to research ethics if we just used normal saline injection in the control group. Therefore, conservative treatments were prescribed to all patients of both groups as follows: Oral celecoxib 100mg tab BID for 4 weeks, modifying physical activity and performing physiotherapy periodically (15 sessions/3 times a week), and prohibition of taking other NSAIDs.
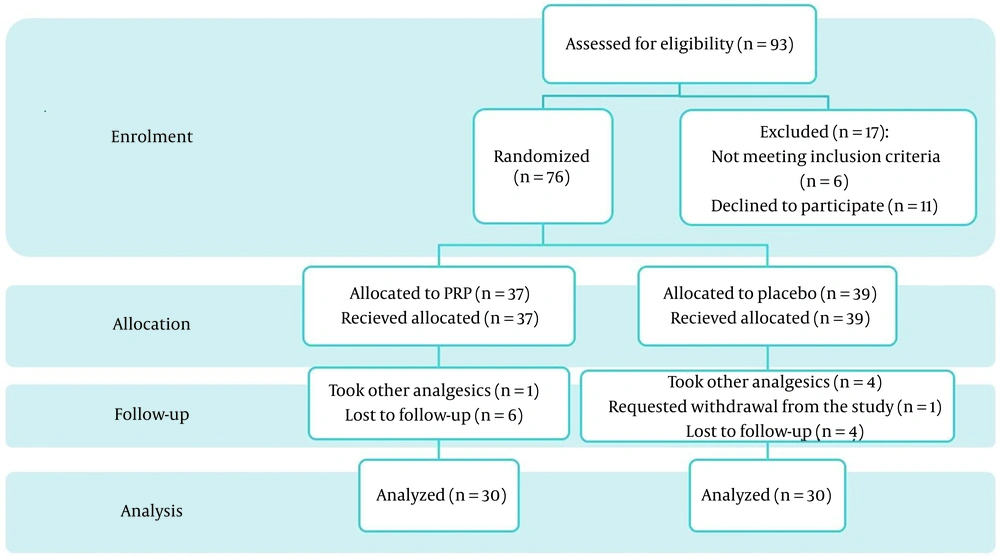
Enrollment was performed based on the per-protocol method, and randomization was applied using random block design (block size: 4). For patients in the intervention group, intra-articular injection of PRP, in addition to conservative treatment were prescribed. In the control group, an intra-articular injection of normal saline (placebo) plus conservative treatment was applied (Figure 1).
3.3. The Procedure of PRP Preparation
For each patient, a sterile kit containing a specific needle and 4 potassium citrate (3.2%) vacutainer tubes were used. Briefly, 10 mL of blood was taken and spilled equally into the vacutainer tubes with careful attention to prevent any damage to the platelets. Tubes were shaken gently to mix blood and anticoagulant completely. Tubes were centrifuged at 3200 rpm for 12 min. Then, the plasma and buffy coat were slowly transferred to another tube. To increase the platelet concentration, tubes were again centrifuged using 2400 rpm for 5 min. The obtained PRP was utilized for intra-articular injection. To prevent any differences in the protocol of the study between the two groups, blood sampling from the control group was also performed in a similar manner, but the concentration and injection steps were not performed. Instead, normal saline as a placebo was injected with a similar protocol. All steps of preparation and filling of the syringe were performed away from the patient, the syringe was covered during the injection, and the patient was asked not to look at the injection site.
3.4. Functional Outcomes
The VAS score and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) were measured pre-treatment, at the 1st week, and at the 3rd and 6th months after the end of the medical and physical treatments. We have used the validated Persian version of WOMAC questionnaire, which is an international standard questionnaire for the evaluation of the therapeutic outcomes in patients with knee osteoarthritis. Each question has 5 choices with a score of 0 - 4 for each response, and the total score was ranked between 0 - 100. The reduction in the WOMAC score is a symptom of improvement (13-15).
3.5. Statistical Analysis
All data, including age, gender, weight, BMI, and scores obtained from VAS and WOMAC questionnaires, were inserted in the patient’s data form and analyzed using SPSS 19 software (IL, Chicago, USA). Categorical variables were presented as frequency and percentage, and numerical variables were presented as mean and standard deviation. An independent t-test was applied to compare WOMAC and VAS scores between the two groups. Repeated measure ANOVA was employed to evaluate the trend changes of quantitative variables (VAS and WOMAC scores). To determine the effects of underlying variables such as age, gender, and BMI on the therapeutic outcomes, analysis of covariance (ANCOVA) was used. P < 0.05 was considered a significant difference.
4. Results
Ninety-three patients were initially eligible for the study. Per-protocol sampling was done until 30 patients were enrolled, allocated, and analyzed completely in each group. During the follow-up, six patients in the intervention group and four patients in the control group were lost-to-follow-up, and the number of people who used other analgesics or NSAIDs during the study was 1 and 4 patients in the intervention and control group, respectively (Figure 1).
The total mean age of patients was 61.6 ± 6.2 years (age range 40 - 65 years), and 65% (39 cases) were female. Demographic characteristics (age, gender, and BMI) were not significantly different between the two groups (Table 1). Also, functional scores of patients (VAS and WOMAC) were similar between the two groups before the treatment (P > 0.05).
| Variables | PRP | Control | Level of Significant |
|---|---|---|---|
| M/F (No.) | 12/18 | 9/21 | N.S. |
| Age (mean ± SD) | 62.6 ± 5.9 | 60.6 ± 6.3 | N.S. |
| BMI | 29.7 ± 3.1 | 29.1 ± 2.7 | N.S. |
| Baseline VAS | 5.9 ± 1.1 | 6.3 ± 1.3 | N.S. |
| Baseline | 54.9 ± 4.8 | 53 ± 4.3 | N.S. |
Abbreviations: PRP, platelet-rich plasma; BMI, body mass index; WOMAC, western ontario and mcmaster; N.S., no significant.
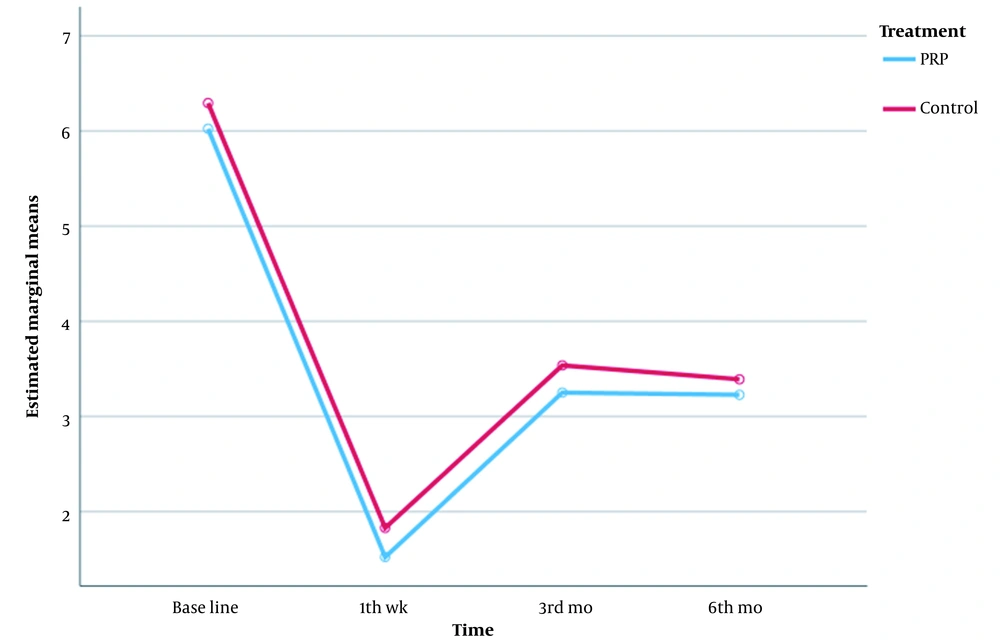
It was found that patients in both groups responded more significantly to the treatment in the first week after treatment (Figure 2). In the intervention group, in the third and sixth months, the mean change chart demonstrated a plateau, and no clear changes were seen in the rate of pain reduction. Significant changes in the rate of pain reduction in the third and sixth months were not found in the control group either. A significant reduction in the pain score in the first week after treatment was seen in both groups. In the comparison of the VAS scores, it was found that mean change of pain reduction was statistically not significant between the intervention and control groups. It means that PRP + conservative treatment was effective, similar to normal saline + conservative treatment (Table 2 and Figure 2).
| Descriptive Statistics | Treatment (Mean ± SD) | P-Value |
|---|---|---|
| VAS, before | 0.294 | |
| PRP | 6.02 ± 1.210 | |
| Control | 6.29 ± 1.146 | |
| VAS-1 wk | 0.237 | |
| PRP | 1.52 ± 1.151 | |
| Control | 1.83 ± 1.223 | |
| VAS-3 mo | 0.243 | |
| PRP | 3.25 ± 1.164 | |
| Control | 3.54 ± 1.075 | |
| VAS-6 mo | 0.495 | |
| PRP | 3.23 ± 1.097 | |
| Control | 3.39 ± 1.093 | |
| P-value | ||
| Time < 0.001 partial Eta square of time | 0.810 | |
| Observe power | 0.999 | |
| Group | 0.159 | |
| Interaction of time and group | 0.825 |
Abbreviation: PRP, platelet-rich plasma.
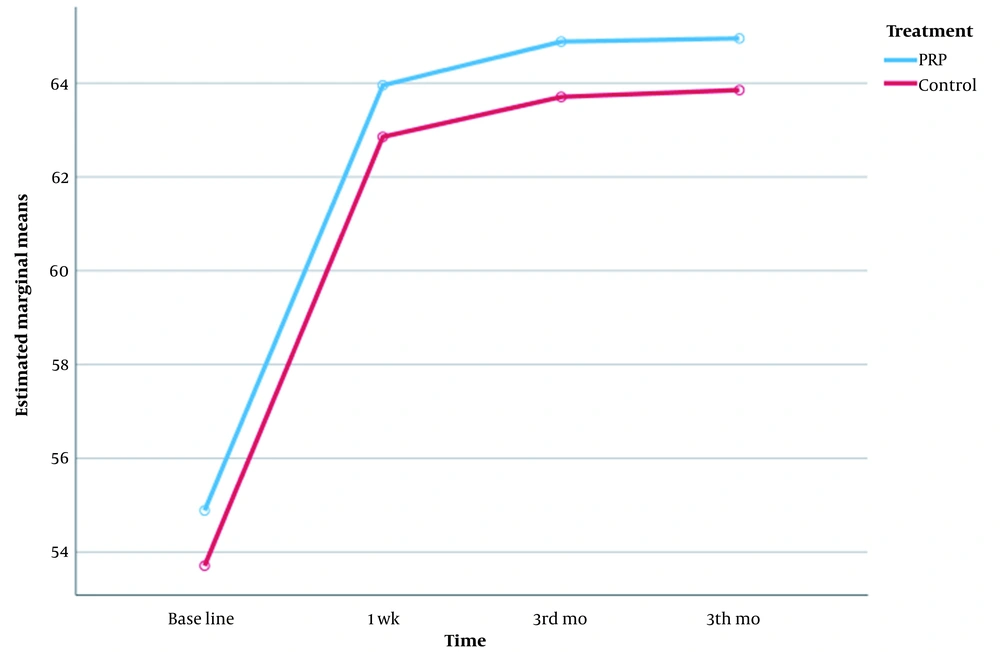
In addition, the WOMAC score was improved from pre-treatment to the first week after treatment in both groups. However, this index did not show any progress in the subsequent follow-up in the third and sixth months in both groups and remained at the same values of the first week. This indicates that the improvement trend in both groups was incremental in the first week, but in the third and sixth months, there was no clear change. In the comparison between the two groups, the mean changes in both groups were similar, and both treatments had been, to some extent, effective in the improvement of articular function (Table 3 and Figure 3).
| Descriptive Statistics | Treatment (Mean ± SD) | P-Value |
|---|---|---|
| WOMAC, before | 0.107 | |
| PRP | 54.89 ± 3.479 | |
| Control | 53.71 ± 3.180 | |
| WOMAC-1 wk | 0.105 | |
| PRP | 63.95 ± 3.797 | |
| Control | 62.85 ± 3.972 | |
| WOMAC-3 mo | 0.107 | |
| PRP | 64.89 ± 3.479 | |
| Control | 63.71 ± 3.180 | |
| WOMAC-6 mo | 0.195 | |
| PRP | 64.95 ± 3.797 | |
| Control | 63.85 ± 3.972 | |
| P-value | ||
| Time < 0.001 partial Eta square of time, observe | 0.904 | |
| Power | 0.999 | |
| Group | 0.120 | |
| Interaction of time and group | 0.898 |
Abbreviation: PRP, platelet-rich plasma.
Changes of VAS and WOMAC scores with adjustment of the underlying variable of gender using t-test in the first week, third and sixth months were not significant (Table 1).
5. Discussion
The highest decrease in pain severity and the greatest increase in WOMAC index were in the first week post-treatment. Albeit, the mean change of pain intensity was approximately similar in comparison between the two groups. This means that both treatments have been effective in reducing pain equally without significant differences. Overall, no superiority was seen in PRP + conservative against the normal saline + conservative group. Two underlying factors, age, and BMI, showed a significant effect on the treatment outcome. In the patients with lower BMI, response to the treatment by PRP was significantly better than the control group. Also, the age variable was effective, and patients with lower age demonstrated better response to treatment by PRP + conservative in comparison to normal saline + conservative treatment.
In recent years, several studies have been published about the evaluation of the therapeutic effects of PRP by different methods. Differences in studies can be divided into several categories, including PRP preparation method (single or double spinning), cellular content (leukocyte-poor or leukocyte-reach PRP), number of injections (single or multiple injections), and the number of replicates and the time interval (in case of applying multiple injections). However, researchers have not reached a standard consensus (2, 8, 16). In this study, a single injection of double-spinning leukocyte-poor PRP was used.
Some studies have demonstrated the negative pro-inflammatory effects of leukocytes that may lead to exacerbating the transient catabolic pathway and degenerating articular surfaces (17). But on the other hand, studies have shown that leukocyte-reach PRP is similar to leukocyte-poor PRP with regard to therapeutic outcomes and security profile (18).
Platelets’ alpha-granules produce and secrete certain growth factors, including platelet-derived growth factors (PDGF). These factors may improve chondral remodeling. Since the degenerative changes in osteoarthritis outweigh the regenerative joint process, these factors may be able to increase the production of chondrocytes and articular matrix in the long term and, therefore, may liken regeneration rate to degradation level (12). Vascular endothelial growth factors may also play a chondroinductive role (19). On the other hand, dramatic pain relief after PRP injection may be correlated with the down-modulation of the inflammation. Regulation of the cyclooxygenase-2 (COX-2) may lead to the arrangement of the anti-inflammatory cascade and alleviate pain in the early weeks after the injection (20).
Another point that can be considered is that the experimental effects of PRP are optimal and acceptable. Rationally, platelets have several growth factors, the release of which into the articular tissue leads to cellular production, chemotaxis, and modulation of the inflammatory response (21-23). However, the clinical outcomes are still far from the laboratory ones, and which of the preparation techniques and injection protocols provides better results is unknown. Simply, meta-analyses and systematic reviewers have failed to make a confirmed conclusion due to this diversity in the protocol of different studies, as well as other biases, confounding factors, and other underlying variables (2, 16, 24). For instance, in the present study, it was found that lower age and lower BMI had an increased effect on the treatment’s response. Filardo et al. also concluded in his research that younger people showed a better response to PRP (25). Cole et al. compared HA and leukocyte-poor PRP in their recent study with 111 patients. They found that in patients at milder stages of osteoarthritis and patients with lower BMI, the response to PRP was better (26). Spakova et al., in 3 comparisons of HA and PRP, concluded that the therapeutic effects of PRP were better in the early stages of OA [grade 1, 2, 3 Kellgren (27)]. Albeit, there is some controversy. For instance, Cerza et al. (28) did not find a relationship between OA grade and healing rates after PRP injection, and Patel et al., in their study, did not find any effect of age, weight, and BMI on the healing rate (12). Totally, it seems that not only does the design of the PRP therapy technique have a direct effect on the outcomes, but the effect of underlying factors is not less than that. Also, in a pilot study in 2010, Sampson et al. found that people who are younger and have lower degrees of osteoarthritis respond better to PRP injections (29).
Obesity is an abnormal stress, and aging is an abnormal physiology; both of which play a mechanical loading role. This pressure results in degradation of the matrix, increased catabolic activity, and aberrant repair response (30). Lower inflammatory responses in younger patients and those with lower BMI may be the possible reason for better response and more effective healing after PRP injections (30).
Pathophysiologically, ligament laxity is increased with aging and causes knee joint instability. On the other hand, the articular muscles are also weakened and become more atrophic with increasing age (31). As a result, the pressure on the germinal and viable cells of the intra-articular parts is increased and disrupts their function more than before. Therefore, it can be concluded that in people of lower age, the pressure on this germinal and repairing part of the joint is less. Thus, treatment with PRP becomes more helpful (31).
If the therapeutic results of PRP are divided into two parts chronologically, the first part can be associated with its anti-inflammatory effects, and longer-term results are related to chondral remodeling effects (11, 32). However, long-term responses in different studies do not seem to be as promising as short-term results. In this study, the response to treatment in the first week was remarkable, and the six-month results remained the same.
One of the limitations of this study was the short duration of follow-up which forced us to evaluate short-term outcomes. Therefore, we cannot conclude about the long-term beneficial or adverse effects of PRP usage. The difference in the satisfaction of patients with osteoarthritis can be a result of sex-related biological and biochemical differences, differences in perception, and the threshold of pain in different patients, which affected the functional scores. Other effective factors are the job and the level of individuals’ activities which could not be controlled in this study as underlying variables and can be considered in future studies. The strengths of this study include appropriate population, randomization, involvement of a control group, blindness, and consideration of certain underlying variables such as age, sex, and BMI. Moreover, due to the existence of several PRP treatment protocols, which induced difficulty in finding a standard protocol, it is better to design and perform more studies with higher quality.
5.1. Conclusions
Platelet-Rich Plasma can be an appropriate choice for the treatment of moderate knee osteoarthritis, especially in younger patients and those with lower BMI.