1. Background
Oral candidiasis (yeast-like fungus) is an opportunistic fungal infection of the oral mucosal membranes mainly caused by Candida (1). Oral candidiasis is most often caused by Candida albicans and, to a lesser extent, by C. parapsilosis, C. tropicalis, C. glabrata, C. krusei, C. pseudotropicalis, and C. guilliermondii (2).
Various chemical agents, including alkaline peroxidase, acids, enzymes, toothpaste, and mouthwashes, have been introduced into clinical use to manage C. albicans (3). Chlorhexidine (CHX) is an antiseptic agent with broad-spectrum antibacterial activity against various microorganisms, including viruses, bacteria, and fungal species such as C. albicans (4). Due to its clinical efficacy, CHX is the gold standard for assessing other antibacterial agents (5). In addition to wide-spectrum antimicrobial activity, CHX has been shown to have several side effects, including tooth staining, unpleasant taste, mouth dryness, mouth irritation, and hypersensitivity reactions (5). Moreover, long-term use of antifungal drugs and chemical drug interactions have resulted in drug resistance, especially in immunocompromised patients (6).
Because of the adverse effects of CHX and the abovementioned problems, attempts have been made to introduce and test new antimicrobial agents. Herbal medicines have long been used worldwide as complementary and alternative choices to chemical drugs (6). Various herbal agents have already been introduced as mouthwashes. The global request for herbal medications has significantly increased. Therapeutic effects, affordability, little to no side effects, and no microbial resistance are among the advantages of herbal medicines (7).
Some herbal mouthwashes have already been tested. Matrica (Kamisol®) (Poursina, Tehran, Iran) is an herbal mouthwash containing an aqueous extract of chamomile and is used in various bacterial infections and oral diseases. Matrica mouthwash has antibacterial, antifungal, and antiviral activities (8, 9).
Persica (Poursina, Tehran, Iran) is another herbal mouthwash designed and prepared with the alcoholic extract of Salvadora persica (the toothbrush tree). Persica has been shown to have widespread benefits for human health, including antiplaque, anti-ulcer, antihemorrhagic, analgesic, and antimicrobial properties (5, 9-13). Moreover, Persica can potentially prevent dental caries and treat gingival and periodontal diseases without side effects (5, 9-13). However, its efficacy is still a matter of debate (14).
Recently, 2 new types of mouthwash have been introduced; however, their antifungal effects remain completely unknown. Cinnamol (Goldaru, Isfahan, Iran) is a commercial herbal mouthwash that contains hydro-alcoholic extracts of cinnamon and cardamom and flower buds of the clove tree. With its deodorant properties, Cinnamon has been established to possess pharmacological and antibacterial activities (15). However, its antifungal activity has not been studied yet.
Jaftex is an experimental, patented herbal mouthwash containing an aqueous oak fruit hull (jaft) extract as the base and marine extracts of Zataria multiflora and Satureja bachtiarica. The antimicrobial activity of the Jaftex mouthwash on common oral microorganisms such as Streptococcus mutants, S. salivarius, and S. sanguinis has been confirmed (16, 17). However, its antifungal effects have not been documented yet.
2. Objectives
This study was conducted to comparatively evaluate the antifungal effects of these 4 herbal types of mouthwash (2 novel herbal drops of mouthwash [Cinnamol and Jaftex] and 2 others [Matrica and Persica]) v. CHX on the growth of C. albicans and C. glabrata.
3. Methods
The present in vitro study was conducted in 2021 in the Department of Microbiology, School of Medicine, Ahvaz Jundishapur University of Medical Sciences (AJUMS), Iran. All the experiments were performed as unique experiments with at least 3 repetitions. The Ethics Committee of AJUMS approved the study procedure (ref. no. IR.AJUMS.REC.1399.809).
3.1. Candida Preparation
The standard strains of C. albicans (PTCC5027) and C. glabrata (ATCC 90030) were prepared as lyophilized ampoules (inactive and powdered) from the Culture Collection and Microbial Resources Center (Tehran, Iran).
3.2. Sampling, Isolation, Cultivation, and Characterization of Specimens
A volume of 2 mL of the subdextrose culture medium (Merck, Merck KGaA, Darmstadt, Germany) was placed into lyophilized vials. The vials were mixed with the microbial suspension, set in Sabouraud dextrose agar (SDA) (Merck), and incubated for 24 h at 37°C. The stock culture solutions of each strain were prepared and stored in the freezer at -70°C for reuse. After 24 h of incubation, suspensions with 0.5 McFarland turbidity (1.5 × 108 CFU/mL) were prepared.
3.3. Experimental and Commercial Mouthwashes
Matrica (Poursina, Tehran, Iran), Persica (Poursina), Cinnamol (Goldaru, Isfahan, Iran), and CHX (Jaber Ebne Hayyan, Tehran, Iran) were obtained from an authorized pharmacy.
The experimental Jaftex mouthwash with patent number (139350140003008118) was prepared in the Medicinal Plants Research Center of Ahvaz Jundishapur University of Medical Sciences. The active ingredients of Jaftex included 10 grams of oak fruit (jaft), S. bachtiarica, and Z. multiflora. The extraction process of each plant was as follows: Jaft was washed with distilled water, dried at laboratory temperature, and powdered using an electric herb powder grinder. The prepared powder was poured into a double-layered bag. The bag was placed in an Erlenmeyer flask containing 150 mL of distilled boiling water. The solution was stirred at a low speed for 24 hours at laboratory temperature. After filtering the extract with a 2-layer cloth, the solution was passed through Whatman® Grade 1 filter paper and centrifuged at 2400 rpm for 10 minutes. The resulting transparent liquid was then kept in the dark container at 4°C. Similarly, S. bachtiarica and Z. multiflora extracts were prepared like the jaft extraction method. In the last step, the aqueous extracts of the 3 plants were mixed, and the volume of the solution was increased to 100 mL with distilled water and stored in the refrigerator.
3.4. Antifungal Susceptibility Testing
All the following experiments were done 3 times for each mouthwash-Candida combination (18, 19). The antifungal activity of the studied mouthwashes and the susceptibility of C. albicans and C. glabrata to antifungals were evaluated using the broth macrodilution test. The antimicrobial agent was double-diluted in 8 sterile test tubes (double serial dilutions of test tubes). Eight standard sterile test tubes were numbered. Then, a sampler poured 1 mL of distilled water into tubes 2 to 8. In the next step, 2 mL of the mouthwash was poured into tube 1, and 1 mL was taken from tube 1 and poured into tube 2. Tube 2 was shaken, and 1 mL was taken from it and poured into tube 3. This process continued until the main concentration was reduced by half. Hemolysis glass tubes are used in the macrodilution method. The final volume is 1 mL in the macrodilution process. The serially diluted fungal suspension (5 × 106 CFU per mL of the liquid culture medium) was added to the diluted antifungal mouthwash with the same volume at a ratio of 1:1. All the test tubes were incubated at an appropriate growth temperature (37°C) for 24 hours. The growth of the microorganisms in the tubes was inspected with the naked eye.
The mouthwash concentration (weight of mouthwash in 1 cc of the solvent expressed as a percentage) in the last tube, which was clear and exhibited no visible fungal growth, was considered the minimum inhibitory concentration (MIC) (%).
The minimum fungicidal concentration (MFC) was determined by transferring the content of transparent tubes to solid culture media containing SDA: 10 μL of the solution was picked up from each transparent tube with a sampler and transferred to plates containing solid SDA with chloramphenicol, and incubated for 24 hours at 37°C. The lowermost concentration of the mouthwash that prohibited the development of either strain of Candida on the SDA medium was considered MFC.
The whole set of tests was performed in triplicate for each mouthwash as a standard number for reliable antimicrobial testing (18, 19).
3.5. Statistical Analysis
The sample size for each complete experiment on each mouth rinse on each Candida strain was determined as 3, following the standards for antimicrobial observations (18, 19). Therefore, 15 observations for each of the 2 Candida strains were performed, accounting for a sample size of n = 30. Descriptive statistics were calculated. The Kruskal-Wallis test, followed by the Dunn-Bonferroni post-hoc test, was used to compare the MIC and MFC of Cinnamol, Jaftex, Matrica, Persica, and CHX mouthwashes on either Candida strain. These comparisons were repeated only among the herbal mouthwashes after excluding CHX. The software used was SPSS v. 25 (IBM, Armonk, NY, USA). The significance level was set to 0.05.
4. Results
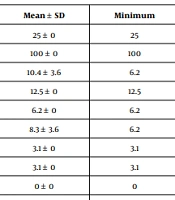
The descriptive statistics for the MIC and MFC of the studied mouthwashes against the strains of C. albicans and C. glabrata are presented in Table 1.
| Candida | Mouthwash | Variable | Mean ± SD | Minimum | Median | Maximum |
|---|---|---|---|---|---|---|
| albicans | Jaftex | MIC | 25 ± 0 | 25 | 25 | 25 |
| MFC | 100 ± 0 | 100 | 100 | 100 | ||
| Persica | MIC | 10.4 ± 3.6 | 6.2 | 12.5 | 12.5 | |
| MFC | 12.5 ± 0 | 12.5 | 12.5 | 12.5 | ||
| Matrica | MIC | 6.2 ± 0 | 6.2 | 6.2 | 6.2 | |
| MFC | 8.3 ± 3.6 | 6.2 | 6.2 | 12.5 | ||
| Cinnamol | MIC | 3.1 ± 0 | 3.1 | 3.1 | 3.1 | |
| MFC | 3.1 ± 0 | 3.1 | 3.1 | 3.1 | ||
| Chlorhexidine | MIC | 0 ± 0 | 0 | 0 | 0 | |
| MFC | 0.2 ± 0.1 | 0.1 | 0.1 | 0.3 | ||
| glabrata | Jaftex | MIC | 12.5 ± 10.8 | 6.2 | 6.2 | 25 |
| MFC | 25 ± 21.6 | 12.5 | 12.5 | 50 | ||
| Persica | MIC | 5.2 ± 1.8 | 3.1 | 6.2 | 6.2 | |
| MFC | 10.4 ± 3.6 | 6.2 | 12.5 | 12.5 | ||
| Matrica | MIC | 5.2 ± 1.8 | 3.1 | 6.2 | 6.2 | |
| MFC | 10.4 ± 3.6 | 6.2 | 12.5 | 12.5 | ||
| Cinnamol | MIC | 0.7 ± 0 | 0.7 | 0.7 | 0.7 | |
| MFC | 1.5 ± 0 | 1.5 | 1.5 | 1.5 | ||
| Chlorhexidine | MIC | 0 ± 0.1 | 0 | 0 | 0.1 | |
| MFC | 0.13 ± 0.2 | 0 | 0 | 0.3 |
Descriptive Statistics of Minimum Inhibitory Concentration and Minimum Fungicidal Concentration Values (%) of the Studied Mouthwashes Against the Strains of C. albicans and C. glabrata.
4.1. C. albicans
There was a significant difference across the MIC of the 5 studied mouthwashes against C. albicans (P = 0.008). Also, there was an overall significant difference among the mouthwashes regarding the MFC of C. albicans (P = 0.009, Kruskal-Wallis). The lowest MIC and MFC values against C. albicans were reported for CHX. In contrast, the highest MIC and MFC values against the strains of C. albicans and C. glabrata were observed in the Jaftex mouthwash (Table 1). The only Dunn-Bonferroni pairwise comparison between the mouthwashes regarding the MIC of C. albicans was between Jaftex and CHX (P = 0.008). Similarly, the only significant MFC difference was observed between these two (P = 0.008). No significant pairwise MIC or MFC comparison was detected (P > 0.05).
After excluding CHX, the Kruskal-Wallis showed overall significant differences across the 4 herbal drops of mouthwash in terms of both MIC (P = 0.015) and MFC (P = 0.015). The only considerable post-hoc comparisons were between Jaftex and Cinnamol in terms of MIC (P = 0.009) and MFC (P = 0.009), while the rest were nonsignificant (P > 0.05).
4.2. C. glabrata
The overall difference among the MIC of the 5 studied types of mouthwash against C. glabrata was significant (P = 0.015, Kruskal-Wallis). Also, there was a significant MFC difference across the mouthwashes regarding C. glabrata (P = 0.015, Kruskal-Wallis). The lowest and highest MIC or MFC values against C. glabrata were seen in CHX and Jaftex mouthwashes, respectively (Table 1). The only pairwise comparison between the MIC of C. albicans was between Jaftex and CHX (P = 0.024). Regarding MFC, the only significant post-hoc comparison was between Jaftex and CHX (P = 0.024). No other pairwise MIC or MFC comparison was seen (P > 0.05).
After excluding CHX, the Kruskal-Wallis showed overall significant differences across the 4 herbal mouthwashes in terms of both MIC (P = 0.039) and MFC (P = 0.039). The only considerable post-hoc comparisons were between Jaftex and Cinnamol in terms of MIC (P = 0.030) and MFC (P = 0.030), while the rest were nonsignificant (P > 0.05).
5. Discussion
The present study compared the antifungal effect of 4 herbal types of mouthwash (Cinnamol, Jaftex, Matrica, and Persica) against CHX on the growth of C. albicans and C. glabrata. The results of this study suggested that the highest antifungal activity belonged to CHX, followed by Cinnamol, Matrica, Persica, and Jaftex, respectively.
Giuliana et al. (20) studied the antifungal properties of 7 commercial types of mouthwash against Candida species. They suggested that chlorohexidine was more effective against C. albicans (20), which was consistent with the results of the present study. Similarly, Talebi et al. (4) reported that CHX had an inhibitory effect on strains of C. albicans; however, its effectiveness was less than that of the other mouthwashes studied (4). Similarly, in this study, CHX inhibited the growth of C. albicans.
In the present study, all the studied mouthwashes showed antifungal activity, among which Cinnamol showed the highest antifungal activity against the strains of C. albicans and C. glabrata; in this regard, C. glabrata was more susceptible to Cinnamol than C. albicans. Cinnamol contains hydroalcoholic extracts of cinnamon and cardamom and flower buds of the clove tree. The active herbal ingredients of Cinnamol might strengthen its antifungal and antimicrobial properties. Cinnamaldehyde is one of this product's most potent ingredients that prevents Candida species' growth. The antibacterial activity of Cinnamol might be due to the presence of citral and geraniol compounds (21-23).
Since one of the ingredients of Cinnamol is cinnamon, the following studies which address the antifungal activity of cinnamon may be of use. Brochot et al. (24) examined the antibacterial, antifungal, and antiviral activities of 3 essential oils of C. zeylanicum, E. caryophyllus, and O. vulgare and their main compounds (cinnamaldehyde, eugenol, and carvacrol) against Candida strains and verified the fungicidal property of these products against C. albicans and C. glabrata (24). Similarly, the Cinnamol mouthwash showed significant antifungal activity against Candida strains in the present study. The reason could be attributed to cinnamon and cloves compounds. Veilleux and Grenier (25) showed that cinnamon bark oil inhibited the growth of C. albicans and suggested that the antifungal activity of cinnamon oil may be due to its capacity to destroy the cell membrane (25). Fani and Kohanteb (26) examined the antimicrobial activity of cinnamon and eucalyptus oils against oral microorganisms, including C. albicans and C. glabrata, and concluded that cinnamon oil showed significant inhibitory activity against oral microorganisms, which was consistent with the results of the present study (26). de Almeida et al. (27) evaluated the efficacy of 2 essential plant oils from Cymbopogon winterianus (citronella) and Cinnamon cassia (cinnamon) against C. albicans biofilms; they concluded that citronella and cinnamon essential oils have anti-C. albicans activity and can be used for daily denture cleansing (27). Yanakiev (28) reviewed the antimicrobial activity of cinnamon essential oil, cinnamon extracts, and pure compounds against different oral pathogens and the oral biofilm. They concluded that cinnamon essential oil, cinnamon extracts, and pure compounds have the potential to inhibit the growth of oral pathogens and prevent dental caries and periodontal disease (28). Varadarajan et al. (29) evaluated the antimycotic activity of hydroalcoholic extracts of Trigonella foenum-graecum seeds, Cinnamomum verum bark, and Carica papaya leaves and seeds against fluconazole-resistant C. albicans; they asserted that all the studied medicinal plants could be used as an alternative medicine to manage fluconazole-resistant candidiasis (29). In the present study, Cinnamol displayed a potential anti-Candida activity and can be used as an alternative to chemical mouthwashes. Since no study has addressed the antifungal activity of Cinnamol, the present study could pave the way for further experiments and studies.
Apart from Cinnamol, the present study showed that Matrica (containing the extract of Matricaria chamomilla) had better antifungal activity against C. albicans than Persica (containing the extract of S. persica). However, no significant difference was found between the antifungal activity of Persica and Matrica mouthwashes against the strain of C. glabrata. Our results were in line with the literature in terms of the superiority of Matrica. Talebi et al. (3) evaluated the effectiveness of some mouthwashes against C. albicans and suggested that Matrica had a more vigorous antifungal activity compared to Persica (3). Similarly, Talebi et al. (4) examined the effect of herbal and chemical mouthwashes against C. albicans. They showed that Matrica and Persica mouthwashes had the highest (MIC = 0.062 mg/L) and lowest (MIC = 0.093 mg/L) activities against C. albicans, respectively (4). Zare et al. (30) reviewed herbal oral care products. They showed that the antibacterial activity of Matrica might be more substantial than Persica, which was consistent with the present study's results (30).
In the present study, the Jaftex mouthwash showed better antifungal activity against C. glabrata than against C. albicans. Additionally, Jaftex had the minimum antifungal effect among the studied mouthwashes. Jaftex contains an aqueous oak fruit hull (jaft) extract as the base and marine extracts of Z. multiflora and S. bachtiarica (31). No study has evaluated the antifungal effect of Jaftex against C. albicans and C. glabrata; however, some antifungal activity of some (but not all) of its ingredients has been addressed in the following studies: Sharifi et al. (32) investigated the antifungal properties of Quercus infectoria gall (Oak) on Saprolegnia fungi and concluded that the hydroalcoholic extract of Q. infectoria gall (Oak) could prevent the growth of Saprolegnia fungi (32). Another ingredient of Jaftex is S. bachtiarica extract. This extract has been assessed before and shows antifungal results. Zomorodian et al. (33) evaluated the antimicrobial activity of essential oils of some medicinal plants, including S. khuzestanica, S. bachtiarica, Ocimum sanctum, Artemisia sieberi, Z. multiflora, Carum copticum, and Oliveria decumbens against common oral pathogens. They concluded that these medicinal plants inhibited the growth of the examined oral pathogens, including C. albicans and C. glabrata (33), which was consistent with the results of the present study. Rohi Boroujeni et al. (34) evaluated the anti-Candida activity of the ethanolic extracts of certain medicinal herbs against C. albicans. They observed that S. bachtiarica Bunge and Thymus daenensis Celak had the highest antifungal activity against C. albicans among all the tested materials (34). Ghasemi Pirbalouti et al. examined the anti-Candida activity of some medicinal plants. They reported that the essential oil of S. bachtiarica and extracts of Scrophularia striata and Ziziphus spina-christi might have significant anti-Candida activity (35). It should be noted that these extracts [such as jaft (oak) extract, etc.] were only similar to some parts of the Jaftex formulation and could not replace Jaftex. Thus, more studies on Jaftex and its antifungal activity are recommended.
5.1. Conclusions
All the studied herbal mouthwashes showed antifungal activity. Among the herbal mouthwashes, the newly introduced Cinnamol mouthwash exhibited the best antifungal activity against C. albicans and C. glabrata, while the experimental herbal mouthwash Jaftex was the weakest. Matrica and Persica had relatively proper and similar antifungal activities. Finally, CHX showed the most potent anti-Candida effect compared to the herbal mouthwashes.