1. Background
COVID-19 has been recognized as a severe respiratory disease pandemic (1). Studies have reported variability in the age and sex of patients affected by COVID-19 worldwide (2-4). COVID-19 not only involved the respiratory system but also caused complications in other organs, including the cardiovascular, hematological, psychological, cutaneous, and nervous systems (5). The coronavirus can invade the central nervous system (CNS) and cause neurological pathologies under certain conditions (6). This disease has been associated with neurological complications in the CNS such as encephalopathy, encephalitis, acute myelitis, cerebrovascular disorders, headache, dizziness, stroke, and epileptic seizures. In addition, several types of peripheral nervous system (PNS) disorders, including cranial nerve involvement (e.g., vestibulocochlear neuropathy, olfactory neuropathy), skeletal muscle injury, and Guillain-Barré syndrome (GBS), have been reported as complications of COVID-19 (7, 8).
Guillain-Barré syndrome is the most common cause of acute or subacute generalized weakness, affecting between 0.8 and 1.9 cases per 100,000 people annually in Europe and North America (9). It is commonly characterized by weakness and areflexia and is treated with plasmapheresis and intravenous immunoglobulin (IVIG) (10, 11). The incidence rate of the syndrome in Iran is reported to be between 2 and 11 cases per 100,000 people (12). Several studies have reported a close relationship between GBS and COVID-19 (13-15), and it accounts for 84.2% of the PNS complications associated with COVID-19 in Iran (9). This syndrome has been more commonly observed in males and elderly individuals. Acute motor and sensory axonal neuropathy (AMSAN) and acute motor axonal neuropathy (AMAN) represent the dominant patterns of nerve involvement. Lower limb weakness, facial weakness, and paresthesia were the core clinical presentations in China, Iran, Europe, and the USA (12).
2. Objectives
3. Methods
3.1. Research Tool
In this retrospective analytical study, we included 143 patients admitted to Imam Reza Hospital of Kermanshah University of Medical Sciences with a diagnosis of GBS between February 2016 and March 2022. Data were collected by a neurology assistant from the electronic clinical records of patients registered in the HIS system. To compare the clinical characteristics of GBS, it was essential to divide the patients into two groups: GBS with COVID-19 and GBS without COVID-19. Since some COVID-19 patients were asymptomatic, it is likely that not all patients were aware of their COVID-19 infection; thus, it is not possible to ensure the true infection rate. We considered the following criteria sufficient for diagnosing COVID-19 infection: (1) A positive COVID-19 PCR test, (2) a documented recent infection, or (3) an abnormal chest CT scan compatible with acute or subacute COVID-19 infection.
Guillain-Barré syndrome was diagnosed based on typical clinical symptoms of motor weakness and areflexia, as well as electromyography-nerve conduction study (EMG-NCS) results. Patients under 15 years old, those with other neuromuscular diseases such as polyneuropathy or myopathy, and those with incomplete data were excluded. This study was approved by the Ethics Committee of Kermanshah University of Medical Sciences.
Ultimately, 143 patients met the inclusion criteria, including 93 non-COVID-19 patients and 50 COVID-19 patients. The following data were extracted from the medical records: Age, gender, type of GBS presentation, interval between GBS onset and COVID-19 infection, pattern of nerve involvement on electrodiagnostic studies, type of treatment, patient condition at discharge (complete recovery, incomplete recovery, unchanged symptoms, worsened, or died), ICU admission, and need for intubation and mechanical ventilation.
3.2. Statistical Analysis
The data were analyzed using SPSS version 27 and are reported as mean ± standard deviation. Other data were reported as frequency. The normality of data was assessed using the One-Sample Kolmogorov-Smirnov test. Normally distributed data were analyzed using the independent t-test, and non-normally distributed data were analyzed using the Mann-Whitney non-parametric test. A P-value < 0.05 was considered statistically significant.
4. Results
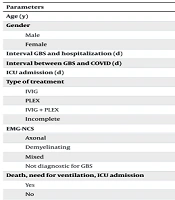
The COVID-19 PCR test result status among the 50 COVID-19 patients showed that 11 patients (7.70%) tested positive, 27 patients (18.90%) tested negative, and 12 patients (8.90%) had unknown results. Half of the patients (n = 25, 50.00%) exhibited symptoms of COVID-19 infection, while the remaining half (n = 25, 50.00%) were asymptomatic. A total of 26 patients (18.20%) demonstrated typical changes of COVID-19 infection on chest CT scans, 16 patients (11.20%) had negative chest CT scans, and 8 patients (5.60%) did not undergo chest CT imaging (Table 1).
| Parameters | COVID | Non-COVID | P-Value |
|---|---|---|---|
| Age (y) | 60.96 ± 12.922 | 50.54 ± 17.895 | 0.001 |
| Gender | 0.069 | ||
| Male | 50.00 | 65.60 | |
| Female | 50.00 | 34.40 | |
| Interval GBS and hospitalization (d) | 71.25 | 72.40 | 0.873 |
| Interval between GBS and COVID (d) | 17.92 ± 15.11 | - | - |
| ICU admission (d) | 74.36 | 69.15 | 0.345 |
| Type of treatment | 0.104 | ||
| IVIG | 38.00 | 52.70 | |
| PLEX | 30.00 | 24.70 | |
| IVIG + PLEX | 12.00 | 11.80 | |
| Incomplete | 20.00 | 7.50 | |
| EMG-NCS | 0.324 | ||
| Axonal | 34.00 | 33.30 | |
| Demyelinating | 26.00 | 34.40 | |
| Mixed | 8.00 | 5.40 | |
| Not diagnostic for GBS | 32.00 | 21.50 | |
| Death, need for ventilation, ICU admission | 0.451 | ||
| Yes | 36.00 | 29.00 | |
| No | 64.00 | No 71.00 | |
| Discharge condition | 0.312 | ||
| Good recovery | 2.00 | 4.30 | |
| Partial recovery | 60.00 | 73.10 | |
| Unchanged | 12.00 | 9.70 | |
| Worsened | 4.00 | 5.40 | |
| Dead | 22.00 | 7.50 |
Comparison of Demographic and Clinical Data in COVID and Non-COVID Patients a
4.1. Demographic and Clinical Characteristics
There was a significant difference in age between patients in the COVID-19 and non-COVID-19 groups (P = 0.001). The mean age was higher in COVID-19 patients compared to non-COVID-19 patients (60.96 ± 12.92 years vs. 50.54 ± 17.90 years, respectively). There was no significant difference between the two groups regarding sex distribution (50% male in the COVID-19 group vs. 65.6% in the non-COVID-19 group, P = 0.069).
The mean interval between GBS onset and hospitalization did not differ significantly between the two groups (71.25 days in COVID-19 patients vs. 72.40 days in non-COVID-19 patients, P = 0.873). The mean interval between COVID-19 infection and GBS onset was 17.92 ± 15.11 days.
The most common clinical symptom in both groups was progressive limb weakness (98% in COVID-19 patients vs. 94.6% in non-COVID-19 patients, P = 0.665). The lowest frequency among 15 different clinical presentations was observed for low back pain in COVID-19 patients (0.00%), and for fecal incontinence, urinary retention, ptosis, and ophthalmoplegia (each 2.2%) in non-COVID-19 patients. Paresthesia was significantly more common in non-COVID-19 patients (P = 0.001). Other symptoms, such as dysphagia, facial paralysis, and ataxia, did not differ significantly between the two groups.
4.2. Treatment and Electro Diagnostic Findings
The majority of patients in both groups were treated with IVIG, with no significant difference in treatment modalities (P = 0.104). Axonal involvement was more prevalent in the COVID-19 group (34%) compared to the non-COVID-19 group (33.3%), while demyelinating patterns were slightly more common in the non-COVID-19 group (34.4%). However, these differences were not statistically significant (P = 0.324).
4.3. Outcome
The patients’ condition at the time of discharge from the hospital did not significantly differ between the two groups (P = 0.312). Partial recovery was observed in 60.00% of COVID-19 patients compared to 73.1% of non-COVID-19 patients. The death rate was 22.00% among COVID-19 patients and 7.50% among non-COVID-19 patients. No significant differences were observed between COVID-19 and non-COVID-19 patients regarding the type of nerve involvement. ICU admission days were comparable between the groups (74.36 days in COVID-19 patients vs. 69.15 days in non-COVID-19 patients, P = 0.345). Additionally, no significant differences were observed regarding the need for mechanical ventilation and ICU admission (P = 0.451).
Table 2 presents the clinical characteristics of patients with COVID-19 compared to those without COVID-19.
| Clinical Presentation | COVID | Non-COVID | P-Value |
|---|---|---|---|
| Progressive limb paralysis | 49 (98.00) | 88 (94.60) | 0.665 |
| Inability to walk | 43 (86.00) | 72 (77.40) | 0.218 |
| Limb paresthesia | 4 (8.00) | 30 (32.20) | 0.001 |
| Dysphagia | 9 (18.00) | 7 (7.50) | 0.058 |
| Dysarthria | 1 (2.00) | 6 (6.50) | 0.422 |
| Dyspnea | 7 (14.00) | 14 (15.10) | 0.865 |
| Weakness of neck muscle | 1 (2.00) | 3 (3.20) | 1.000 |
| Facial paralysis | 2 (4.00) | 10 (10.80) | 0.216 |
| Ptosis and ophthalmoplegia | 1 (2.00) | 2 (2.20) | 1.000 |
| Ataxia | 6 (12.00) | 10 (10.80) | 0.821 |
| Myalgia | 1 (2.00) | 9 (9.70) | 0.165 |
| Low back pain | - | 7 (4.30) | 0.298 |
| Urinary retention | 1 (2.00) | 2 (2.20) | 1.000 |
| Urinary incontinency | 3 (6.00) | 5 (5.40) | 1.000 |
| Fecal incontinency | 2 (4.00) | 2 (2.20) | 0.612 |
Comparison of Clinical Presentation in COVID and Non-COVID Patients a
5. Discussion
Our findings revealed that, apart from the difference in age (P = 0.001), most clinical features, treatment outcomes, and electrodiagnostic patterns were similar between the two groups. This aligns with previous studies, such as those by Palaiodimou et al. (18) and Abu-Rumeileh et al. (15), which reported that older individuals are more susceptible to COVID-19-associated neurological complications, including GBS. The higher age observed in COVID-19 patients could be attributed to the virus's known impact on older adults due to factors such as immunosenescence and the increased prevalence of comorbidities.
Despite this age difference, no significant variation was observed in gender distribution, consistent with studies by Caress et al. (19) and Aladawi et al. (20), which found no strong gender predisposition for GBS associated with COVID-19. Although some reports suggest a male predominance in both COVID-related and classic GBS cases, our findings did not reflect this trend significantly.
In terms of clinical presentation, progressive limb weakness remained the most common symptom in both groups, which is typical for GBS. Interestingly, paresthesia was significantly more prevalent in non-COVID-19 patients (P = 0.001). This contrasts with studies such as Shamim et al. (21), where sensory symptoms were reported equally in both groups. The subjective nature of paresthesia and potential biases in patient history documentation might explain this discrepancy.
Regarding electrodiagnostic findings, the predominance of axonal involvement in the COVID-19 group compared to demyelinating patterns in the non-COVID-19 group was notable but not statistically significant. This trend is partially supported by studies from regions like South Asia, where axonal variants (AMAN and AMSAN) are more common [Bano et al. (22); Yadav et al. (23)]. However, Filosto et al. (24) reported a higher prevalence of demyelinating forms in European COVID-related GBS cases. These variations could be influenced by geographical, genetic, and methodological factors, including the timing of EMG-NCS testing.
Treatment outcomes showed no significant differences between the groups, with most patients receiving IVIG and demonstrating partial or complete recovery. This finding aligns with prior studies [Caress et al. (19); Martinelli-Boneschi et al. (25)], suggesting that standard GBS treatments remain effective regardless of COVID-19 status. However, the mortality rate was slightly higher in COVID-19 patients (22% vs. 7.5%), though not statistically significant. Some studies, such as Bentley et al. (26), have reported higher ICU admissions and ventilation needs in COVID-19-associated GBS, but our data did not reflect these differences, possibly due to sample size limitations or variations in healthcare resources.
Another important observation was the interval between COVID-19 infection and GBS onset, averaging around 17.92 ± 15.11 days. This finding aligns with the typical post-infectious latency period reported in the literature [Sriwastava et al. (27)], supporting the hypothesis that immune-mediated mechanisms play a role in COVID-19-associated GBS.
5.1. Conclusions
In conclusion, while COVID-19-associated GBS was more common in older individuals, other clinical features, treatment responses, and outcomes were similar to those of GBS patients without COVID-19. Future large-scale, prospective studies with standardized outcome measures are needed to better understand the relationship between COVID-19 and GBS and to determine possible causality.
5.2. Study Limitations
This study has several limitations:
- Retrospective design: Limited access to complete patient records may have introduced bias.
- Incomplete data: Missing information on cerebrospinal fluid analysis and the severity of COVID-19-related lung involvement in some patients.
- Sample size: Although larger than many case series, the sample size still limits the generalizability of the findings.