1. Background
Since December 2019, shortly after detecting the first coronavirus disease 2019 (COVID-19) patient in Wuhan, China, the world has faced a new pandemic caused by a novel Coronavirus (CoV) variant. Coronavirus belongs to the order Nidovirales and has the largest ribonucleic acid (RNA) genome (1, 2). COVID-19 causes a wide range of symptoms, from mild respiratory illness (fever, body aches, dry cough) to severe pneumonia, acute respiratory distress syndrome (ARDS), and death. As the World Health Organization (WHO) has noted, this novel CoV, SARS-CoV-2, has emerged as a global health concern (3, 4).
According to guidelines from WHO and the Centers for Disease Control and Prevention, chest radiography and lung computed tomography (CT) scans are essential diagnostic methods during the pandemic. Additionally, evaluating various biomarkers for prognosis and treatment response in COVID-19 patients has become critical (5, 6). Typically, patients with severe or critical COVID-19 exhibit hematologic changes, including lymphocytopenia and a reduction in monocytes, basophils, and eosinophils, along with a significant rise in leukocytes, neutrophils, and the neutrophil-to-lymphocyte ratio (NLR). These alterations reflect an imbalanced immune response in severe cases (7, 8).
Lymphocytopenia is commonly observed in severe cases and is believed to result from direct viral attack on these cells. In contrast, the elevated levels of leukocytes, neutrophils, and NLR indicate an escalated inflammatory response. Neutrophils, while essential in infection defense, can cause tissue damage and organ dysfunction when excessively elevated. The increased NLR highlights a dysregulated pro-inflammatory and anti-inflammatory balance, making these biomarkers crucial for early detection of severe illness (9, 10).
Additionally, biochemical markers such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, D-dimer levels, extended prothrombin time, and inflammatory markers including lactate dehydrogenase (LDH), pro-BNP, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), are often elevated in patients with severe COVID-19 (7, 8, 11). These biomarkers, expressed in various human cells (heart, liver, muscle, kidney, lung, and bone marrow), are indicators of acute or chronic tissue damage (9-11).
According to most studies, these markers correlate with respiratory function and are predictors of respiratory failure in COVID-19 patients. Elevated inflammatory biomarkers have been associated with mechanical ventilation requirements, ICU admission, and higher mortality. Therefore, they are valuable tools for early detection, respiratory monitoring, and timely intervention (12-14).
Preliminary data show significant differences in inflammatory marker levels between patients with acute and non-acute COVID-19 conditions (15-17). Longitudinal studies have demonstrated the importance of tracking these markers over time to assess disease progression and guide clinical management, emphasizing their predictive value for respiratory failure and overall prognosis in COVID-19 patients.
Biochemical and inflammatory markers are key tools for identifying patients at high risk of severe outcomes. Their regular monitoring enables healthcare professionals to make informed decisions regarding patient care and early intervention.
2. Methods
2.1. Patients and Data Collection
This observational, cross-sectional study evaluated all patients admitted to the ICU from February to June 2020, following a confirmed diagnosis of COVID-19 infection. During admission, clinical data, including sex, age, outcome, and laboratory parameters, were documented.
Based on the patient's clinical condition, age, comorbidities, medical history, medication, vital signs, and symptoms, an internal medicine or infectious specialist decides to hospitalize a patient.
ICU admission was based on at least one of the following criteria:
(1) Respiratory distress with a respiratory rate ≥ 30/min, PiO2/FiO2 ratio < 200, or PaO2 < 60 mm Hg, or SpO2 < 90% with FiO2 > 50%;
(2) Hemodynamic instability with a mean arterial pressure (MAP) < 60 mm Hg, unresponsive to 500 mL intravenous fluid administration, requiring vasopressors or inotropes;
(3) Neurological disorder requiring airway support due to a low level of consciousness;
(4) Multiple organ dysfunction requiring organ support (18).
The definitive diagnosis of COVID-19 was confirmed by SARS-CoV-2 RT-PCR testing and evidence of lung involvement on CT scan.
Inclusion criteria: Definite diagnosis of COVID-19 by RT-PCR and lung CT findings consistent with COVID-19 infection.
Exclusion criteria: Presence of cardiovascular, renal, or liver diseases; hemolytic or megaloblastic anemia; diabetes; or cancer.
2.2. Statistical Analysis
Data were analyzed using SPSS 18.0 software (IBM, Armonk, NY, USA). Quantitative variables were described using the mean ± standard deviation. Qualitative variables were reported using frequency distributions. The chi-square test was used to analyze qualitative variables. Independent t-tests were employed to compare mean values between groups when the data were normally distributed. For abnormally distributed data, the non-parametric Mann-Whitney test (Kruskal-Wallis) was used. A P-value < 0.05 was considered statistically significant.
2.3. Ethics Approval
This research adhered to the principles of the Declaration of Helsinki and was conducted with the approval of the Ethics Committee of Golestan University of Medical Sciences, with ethics code IR.GOUMS.REC.1399.349.
3. Results
After applying the inclusion criteria, 263 ICU-admitted patients diagnosed with COVID-19 were included in this study. Diagnosis was based on a positive RT-PCR test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and CT scan findings indicative of COVID-19 infection (Table 1). The mean age of the patients was 59.38 ± 15.26 years, with the youngest being 23 years old and the oldest being 94 years old. The average hospitalization duration was 9.25 ± 6.9 days.
Among the participants, 51.3% (135 patients) were male, and 48.7% (128 patients) were female. Of the total patients, 120 (45.6%) did not survive, 130 (49.4%) survived, and 13 (4.9%) were still under treatment at the time of data collection. All patients who were under treatment survived. At diagnosis, patients were categorized into three groups: Discharged, under treatment, and non-survivors. Age, gender, and hospitalization duration did not show significant differences between non-survivors and survivors (Table 2).
| Variables | Outcome | P-Value | ||
|---|---|---|---|---|
| Survivors | Under Treatment | Non-survivors | ||
| Gender | 0.16 | |||
| Female | 66 (51.6) | 3 (2.3) | 59 (46.1) | |
| Male | 64 (47.4) | 10 (7.4) | 61 (45.2) | |
| Age (y) | 0.33 | |||
| > 60 | 63 (50.8) | 5 (4) | 56 (45.2) | |
| 40 - 60 | 52 (48.6) | 4 (3.7) | 51 (47.4) | |
| < 40 | 15 (46.9) | 4 (12.5) | 13 (40.6) | |
| Admission days | 0.88 | |||
| > 9.25 | 48 (51.1) | 5 (5.3) | 41 (43.6) | |
| < 9.25 | 82 (45.5) | 8 (4.7) | 79 (46.7) | |
Demographic, Characteristics, and Outcomes of COVID-19 Patients Included in the Study
The outcomes of hospitalized COVID-19 patients were assessed in relation to their serum laboratory marker levels, as detailed in Table 3. Hematologic parameters were examined, revealing a statistically significant relationship between white blood cell (WBC) counts, levels of polymorphonuclear neutrophils (PMNs), lymphocyte percentages, and patient mortality (P-value < 0.05). Specifically, patients with leukocytosis, elevated PMN levels, and lymphopenia exhibited poorer clinical outcomes, while those with lower WBC counts and PMN percentages showed better survival rates.
| Variables and Categories | Outcome | Mean | P-Value b | ||
|---|---|---|---|---|---|
| Survivors | Under Treatment | Non-survivors | |||
| LDH (IU/L) | 812.41 ± 438.6 | < 0.05 | |||
| > 812 | 15 (15.5) | 2 (2.1) | 80 (82.5) | ||
| < 812 | 105 (70) | 10 (6.7) | 35 (23.3) | ||
| WBC (109/L) | 9.71 ± 9.82 | < 0.05 | |||
| > 9.71 | 39 (38.6) | 3 (3) | 59 (58.4) | ||
| < 9.71 | 91 (56.2) | 10 (6.2) | 61 (37.7) | ||
| PMN | 80.23 ± 9.28 | < 0.05 | |||
| > 80.23 | 55 (39.6) | 5 (3.6) | 79 (56.8) | ||
| < 80.23 | 75 (61) | 8 (6.5) | 40 (32.5) | ||
| Lymphocyte | 14.33 ± 8.71 | < 0.05 | |||
| > 14.33 | 71 (67) | 7 (6.6) | 28 (26.4) | ||
| < 14.33 | 59 (37.8) | 6 (3.8) | 91 (58.3) | ||
| PLT (109/L) | 208.52 ± 97.04 | 0.58 | |||
| > 208.52 | 56 (53.3) | 5 (4.8) | 44 (41.9) | ||
| < 208.52 | 74 (49.4) | 8 (5.1) | 76 (48.1) | ||
| Hb (g/dL) | 11.62 ± 1.87 | 0.79 | |||
| > 11.62 | 71 (49.7) | 6 (4.2) | 66 (46.2) | ||
| < 11.62 | 57 (49.1) | 7 (6) | 52 (44.8) | ||
| Urea (mg/dL) | 68.18 ± 57.98 | < 0.05 | |||
| > 20 | 109 (46.4) | 11 (4.7) | 115 (48.9) | ||
| < 20 | 20 (74.1) | 2 (7.4) | 27 (18.5) | ||
| Creatinine (mg/dL) | 1.64 ± 1.5 | 0.08 | |||
| > 1.3 | 32 (39) | 5 (6.1) | 45 (54.9) | ||
| < 1.3 | 97 (53.9) | 8 (4.4) | 75 (41.7) | ||
| Na (mEq/L) | 136.31 ± 5.16 | 0.09 | |||
| > 145 | 1 (7.7) | 0 (0) | 12 (92.3) | ||
| 135 - 145 | 78 (49.4) | 8 (5) | 73 (45.9) | ||
| < 135 | 50 (56.2) | 5 (5.6) | 34 (38.2) | ||
| K (mEq/L) | 4.39 ± 0.77 | 0.47 | |||
| > 5.5 | 8 (40) | 0 (0) | 12 (60) | ||
| 3.5 - 5.5 | 114 (51.1) | 12 (5.4) | 97 (43/5) | ||
| < 3.5 | 7 (38.9) | 1 (5.6) | 10 (55.6) | ||
| FBS (mg/dL) | 185.32 ± 100.83 | 0.13 | |||
| > 100 | 51 (40.5) | 4 (6.7) | 71 (56.3) | ||
| < 100 | 17 (56.7) | 2 (6.7) | 11 (36.7) | ||
| CPK (mcg/L) | 594.34 ± 2409.92 | < 0.05 | |||
| > 120 | 43 (38.4) | 5 (4.5) | 64 (57.1) | ||
| < 120 | 32 (61.5) | 2 (3.8) | 18 (34.6) | ||
| Albumin (g/dL) | 3.46 ± 0.56 | 0.76 | |||
| > 3.5 | 37 (58.7) | 2 (3.2) | 24 (38.1) | ||
| < 3.5 | 31 (59.1) | 3 (5.8) | 18 (34.6) | ||
| AST (IU/L) | 60.51 ± 71.08 | 0.05 | |||
| > 60.51 | 15 (32.6) | 1 (2.2) | 30 (65.2) | ||
| < 60.51 | 54 (54) | 9 (9) | 37 (37) | ||
| ALT (IU/L) | 57.45 ± 81.15 | 0.51 | |||
| > 57.45 | 18 (48.6) | 1 (2.7) | 18 (48.6) | ||
| < 57.45 | 51 (46.8) | 9 (8.3) | 49 (45) | ||
| Alkp (IU/L) | 264.89 ± 239.63 | 0.65 | |||
| > 147 | 54 (48.6) | 9 (8.1) | 48 (43.2) | ||
| 44 - 147 | 14 (45.2) | 1 (3.2) | 16 (51.6) | ||
| < 44 | 0 (0) | 0 (0) | 1 (100) | ||
Laboratory Biomarkers and Outcomes of COVID-19 Patients Included in the Study a
Additionally, significant associations were found between serum levels of urea, creatine phosphokinase, and lactate dehydrogenase and patient outcomes, with all showing statistically significant relationships (all P-values < 0.05). Patients with lower CPK and urea levels were more likely to survive compared to those with higher levels. Aspartate transaminase levels also showed a nearly significant relationship with patient outcomes (P-value = 0.05).
Moreover, a majority of patients with hypernatremia (92.3%), elevated fasting blood glucose levels (56.3%), and increased serum creatinine levels (54.9%) did not survive.
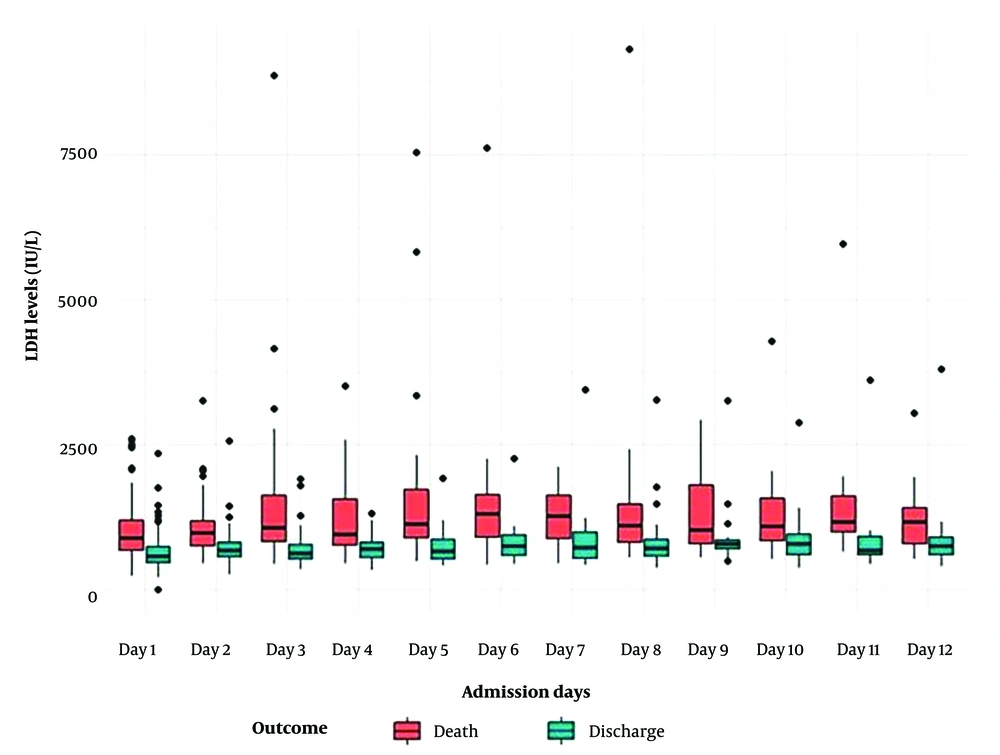
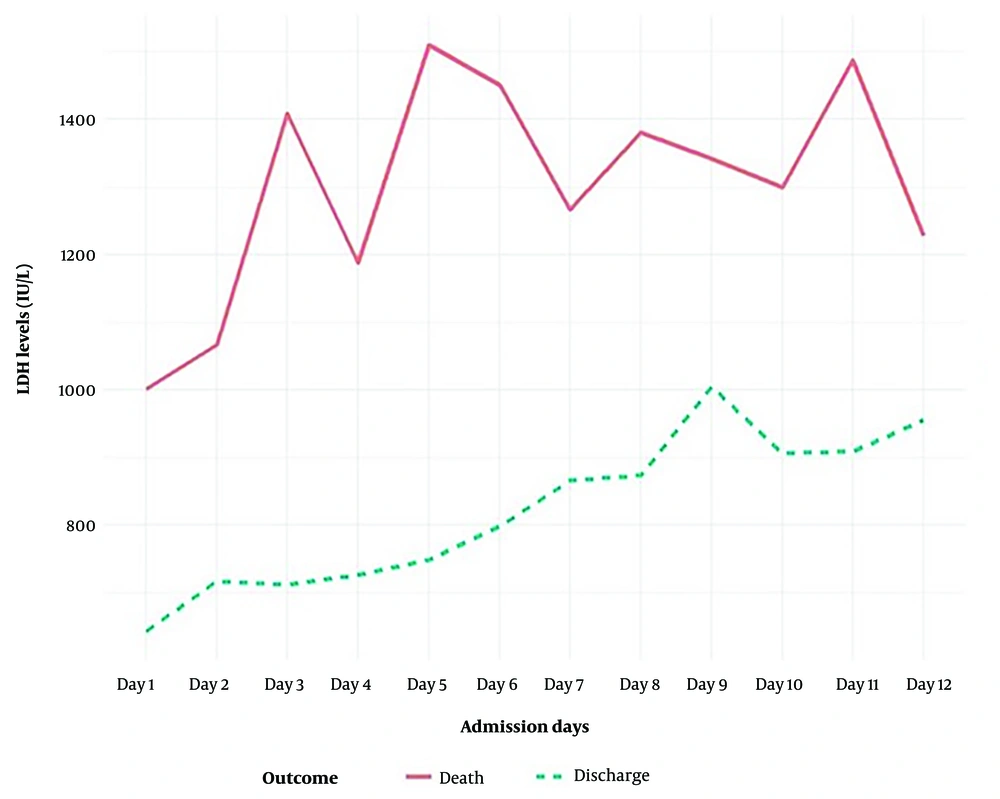
The mean serum LDH level at the time of diagnosis was 812.41 ± 438.6 IU/L. There was a statistically significant difference in LDH levels between the non-survived and survived groups. As the hospitalization duration extended from one to twelve days, serum LDH levels increased. In all cases, non-survivors exhibited higher LDH levels than survivors (Figures 1 and 2).
4. Discussion
COVID-19 is an infectious viral disease typically associated with local or systemic inflammation, which can damage several organs. Despite antiviral treatment and supportive care, the mortality rate from COVID-19 remains high. Organ and tissue damage during COVID-19 can result from excessive inflammation and uncontrolled immune activation. Consequently, inflammatory biomarkers such as neutrophils and CRP, and cellular enzymes like LDH and creatine phosphokinase, are critical indicators for diagnosing, monitoring, and predicting the prognosis of COVID-19 patients.
A study by Poggiali et al. (2020) reported that elevated LDH levels were an independent biomarker for poor prognosis in COVID-19 patients, correlated with respiratory function (PaO2/FiO2), and served as a predictor of respiratory failure (18, 19). Most studies have shown that LDH levels above 200 - 250 IU/L are linked to poor outcomes. Henry et al. (2020) indicated that LDH cut-off values between 245 and 253.2 U/L were associated with a significantly increased risk of mortality. In particular, LDH > 812 IU/L has been identified as a relevant factor in risk assessment for advanced COVID-19 and death. Our study also found that age or sex did not significantly affect the relationship between elevated LDH levels and poor prognosis, though these factors may contribute to increased COVID-19 severity and mortality, potentially confounding the association (17, 19).
Our research further demonstrated that LDH levels rose with the duration of hospitalization, with serum LDH levels increasing as patients’ hospital stays extended from one to twelve days. Elevated LDH has been identified as a powerful predictor for early recognition of lung injury and severe COVID-19 cases (19, 20).
We also observed that leukocytosis and elevated PMN levels were associated with organ damage and hypoxia. Studies have confirmed that these markers reach their highest levels in lung infections and respiratory failure and are linked to poor outcomes and mortality in COVID-19 patients. As such, leukocytosis and elevated PMN levels can be established as key predictive factors for early diagnosis of lung injury and severe respiratory infections, such as COVID-19 (21, 22). Additionally, lymphopenia has been observed in severe COVID-19 cases, further compromising immune responses and increasing vulnerability to secondary infections. Our findings suggest that individuals with lymphopenia are at higher risk of morbidity, mortality, and severe complications from COVID-19 (22, 23).
Elevated urea levels in COVID-19 patients indicate potential kidney damage or dysfunction. Although the exact cause remains unclear, it may result from direct viral effects on the kidneys or dehydration caused by fever, sweating, and reduced fluid intake (24-26).
In severe COVID-19, elevated CPK levels are often seen in patients with complications such as rhabdomyolysis. The cytokine storm syndrome that can occur in severe COVID-19 cases may lead to widespread inflammation, muscle breakdown, and increased CPK levels in the blood. Our research links elevated CPK levels to multi-organ failure, respiratory distress, and increased mortality risk in advanced COVID-19 cases. High CPK levels may indicate muscle damage and inflammation, signaling disease severity and poor outcomes (25-27).
Additionally, our research revealed a relationship between hypernatremia, elevated fasting blood glucose (FBS), and increased serum creatinine levels with higher mortality rates in COVID-19 patients, though these associations were not statistically significant. Hypernatremia may indicate dehydration, leading to organ dysfunction and worsening prognosis, while elevated FBS suggests undiagnosed or uncontrolled diabetes, a known risk factor for severe COVID-19. Elevated serum creatinine may reflect kidney dysfunction, further exacerbating the infection's effects (28-31).
Studies by Letelier et al. (2021) and Ponti et al. (2020) identified elevated levels of inflammatory serum markers (e.g., ESR, CRP, LDH), hepatic markers (AST, ALT), cardiac biomarkers (pro-BNP, CK-MB), and renal function indicators (urea, creatinine) in COVID-19 patients. Patients in more critical conditions exhibited significantly higher levels of these markers, correlating with increased likelihood of organ damage and further elevations in these biomarkers (32, 33).
4.1. Strengths and Limitations
The strength of our study lies in identifying serum biomarkers that help predict disease severity, guide treatment decisions, and monitor COVID-19 progression. By tracking these biomarkers, healthcare providers can gain valuable insights into the disease's underlying pathophysiology and tailor interventions accordingly. Studying biomarkers in ICU patients with COVID-19 provides critical information for improving patient outcomes. However, heterogeneity in results across studies could be attributed to differences in cut-off points, laboratory references, diagnostic tools, underlying diseases, and varying treatment methods. Additionally, variations in sample collection timing among patients could affect result accuracy.
4.2. Conclusions
This study investigated the impact of serum biomarkers on the prognosis of high-risk COVID-19 patients during hospitalization. Our findings suggest that elevated levels of LDH, CPK, urea, WBC, and PMN, as well as lymphopenia, are associated with an increased risk of in-hospital mortality and predict severe respiratory failure in COVID-19 patients. Monitoring these biomarkers during hospitalization could help identify patients at higher risk of adverse outcomes and improve clinical management. Early identification of these biomarkers can aid healthcare providers in stratifying high-risk COVID-19 patients for more intensive monitoring and timely interventions.