1. Background
The emergence of COVID-19 in China in late 2019 led to a rapid global public health crisis. While most patients experienced mild illness and recovered with symptomatic treatment and supportive care, approximately 20% developed severe symptoms requiring hospitalization (1, 2). These patients faced complications that could lead to acute respiratory distress syndrome (ARDS), organ dysfunction and failure, coma, and even death (1, 3). Additionally, individuals with pre-existing chronic health conditions—such as heart disease, diabetes, chronic kidney disease, hypertension, cancer, and pregnancy—were more likely to experience severe manifestations of the infection, resulting in poorer outcomes (4, 5).
COVID-19 infection is associated with a cytokine storm and pulmonary inflammation due to an imbalanced immune response. Patients with severe COVID-19 typically exhibit nonspecific inflammatory responses, including inflammatory cell infiltration, lung edema, thrombosis, and an excessive yet ineffective immune response (6, 7). During the course of the disease, a hyper-inflammatory state develops, characterized by elevated levels of several pro-inflammatory cytokines and chemokines, with interleukin-6 (IL-6) being one of the most predominant (7, 8).
During the pandemic, various strategies using anti-inflammatory medications—particularly corticosteroids, cytokine-targeting antibodies, and JAK inhibitors—were investigated to address harmful hyperinflammation (9, 10). The World Health Organization (WHO) explored potential treatments and recommended systemic corticosteroids, tocilizumab (TCZ) and sarilumab (both IL-6 inhibitors), and baricitinib (a JAK inhibitor), along with a conditional endorsement for remdesivir (an RNA polymerase inhibitor) for patients with severe or critical COVID-19 (11, 12).
Tocilizumab, an IL-6 receptor antagonist, is commonly used for conditions such as rheumatoid arthritis, juvenile idiopathic arthritis, and giant cell arteritis, and has become a key treatment for cytokine release syndrome (CRS) (1, 3, 7). This humanized monoclonal antibody specifically targets the IL-6 receptor and has shown effectiveness in severe SARS-CoV-2 infections by reducing the risk of progression to mechanical ventilation or death (6, 7). Tocilizumab has demonstrated promise in alleviating the severe inflammatory symptoms linked to critical COVID-19 cases, potentially improving clinical outcomes. Studies suggest that early administration of TCZ, especially within the initial 24 hours of ICU admission, can enhance outcomes, including survival rates (6, 9, 11).
Recent findings from randomized controlled trials suggest that TCZ could be beneficial in managing COVID-19 during various waves of the pandemic. However, peer-reviewed data on the clinical use of TCZ in the latest waves are limited, and doubts remain about its effectiveness in reducing mortality in recent waves.
2. Objectives
Therefore, this study aimed to evaluate the clinical outcomes related to the use of TCZ in severe COVID-19 patients during the fifth wave.
3. Methods
3.1. Study Design and Participant Selection
This retrospective cohort study was conducted on COVID-19 patients hospitalized in the intensive care unit (ICU) at Sayyad Shirazi Hospital in Gorgan, Iran, during the fifth wave of the pandemic. Participants were selected from patients admitted to the ICU with severe COVID-19 infections, confirmed through a fluorescent reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) diagnostic test for SARS-CoV-2.
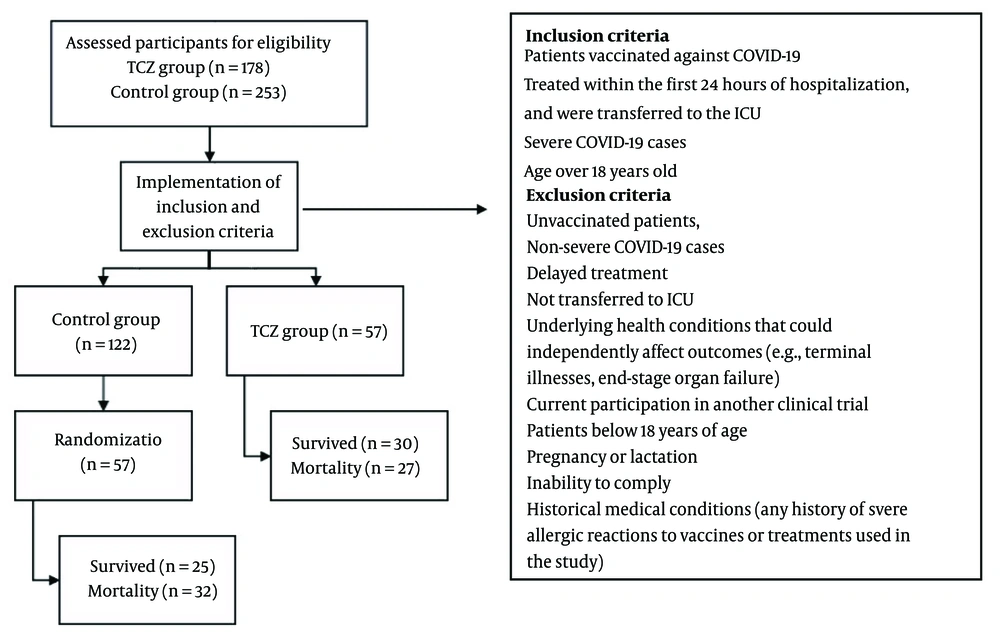
During sampling, the patient population was divided into two groups: Those who received TCZ in accordance with the COVID-19 treatment protocol along with standard treatment (remdesivir and corticosteroids), and those who received only the standard treatment. The first group included 57 patients who received TCZ while hospitalized in the ICU. In the second group, 57 out of the 122 hospitalized patients who received standard treatments were randomly selected for the study.
The inclusion criteria required that patients be vaccinated against COVID-19 per the national vaccination program, diagnosed with severe COVID-19 infection, treated within the first 24 hours of hospitalization, and transferred to the ICU. The exclusion criteria for the study were: Unvaccinated patients, non-severe COVID-19 cases (those with mild or moderate infections), delayed treatment (patients not receiving treatment within the first 24 hours of hospitalization), those not transferred to the ICU, patients with underlying health conditions that could independently affect outcomes (e.g., terminal illnesses, end-stage organ failure), current participation in another clinical trial, patients under 18 years old, pregnancy or lactation, inability to comply, and those with a history of severe allergic reactions to vaccines or treatments used in the study (Figure 1).
Based on the proportion of clinically improved patients receiving TCZ in Vu et al.'s study (13) and calculated using the "two independent proportions in sample size calculation" formula, 57 patients were determined for each study group (α = 0.05, β = 0.2, p1 = 0.639, p2 = 0.361).
3.2. Data Collection
Demographic and clinical data were collected via the patient record system. The patients were divided into two groups: The TCZ treatment group (57 patients) and the standard treatment group (57 patients), serving as the control. The study groups were further divided based on age into two categories: Those under 60 years old and those 60 years or older. Given the increased sensitivity and specificity of the PCR technique for diagnosing infectious diseases, the COVID-19 RT-qPCR test was employed to confirm the diagnosis of COVID-19 in this study.
As per the criteria outlined by the WHO, severe COVID-19 was defined as severe pneumonia, characterized by a respiratory rate exceeding 30 breaths per minute or oxygen saturation levels below 94% on ambient air, the presence of acute respiratory distress syndrome (PaO2/FiO2 < 300), sepsis, or septic shock. The clinical severity of COVID-19 was categorized based on whether patients were treated as outpatients, hospitalized in the general ward, or admitted to the ICU. Ventilatory support included non-invasive mechanical ventilation or orotracheal intubation. The duration of hospital stay was calculated as the number of days from hospital admission to discharge or death (12).
3.3. Statistical Analysis
Data analysis was performed using the Statistical Package for Social Sciences version 25.0 (SPSS Inc, Chicago, IL). Statistical significance was defined as a P-value below 0.05. Results were presented as mean ± standard deviation or standard error. The Shapiro-Wilk test was used to assess the normality of quantitative variables. For normally distributed data, comparisons between groups were made using an independent Student's t-test, assuming homogeneity of variances; otherwise, Welch's ANOVA test was applied. Non-normal distributions were analyzed using the non-parametric Mann–Whitney U test. The chi-square and Fisher's exact tests were employed to evaluate the proportions of qualitative variables.
3.4. Ethics Approval
This study adhered to the principles outlined in the Declaration of Helsinki and received approval from the ethical committee at Golestan University of Medical Sciences under ethics number IR.GOUMS.REC.1401.447.
4. Results
A total of 114 patients were enrolled in the study. The mean age was 53.02 ± 17.29 years in the TCZ group and 53.88 ± 16.71 years in the control group, with no significant difference between the two groups (P-value = 0.34). The proportion of patients aged 60 years or older was 36.8% in both groups, while 63.2% were younger than 60.
In terms of gender distribution, 54.4% of the patients were women and 45.6% were men, with no significant statistical difference between the groups (P-value = 0.25). The frequency of intubation was 3.5% in the TCZ group and 5.3% in the control group, and no significant association was found between the groups (P-value = 0.64) (Table 1).
| Variables | TZC Group (n = 57) | Control Group (n = 57) | P-Value |
|---|---|---|---|
| Age, (y) | 52.16 ± 17.96 | 53.88 ± 16.71 | 0.34 |
| Gender | 0.25 | ||
| Female | 34 (59.6) | 28 (49.1) | |
| Male | 23 (40.4) | 29 (50.9) | |
| Mortality | |||
| < 60 years old | 14 (38.9) | 13 (36.1) | 0.8 |
| ≥ 60 years old | 13 (61.9) | 19 (90.5) | 0.03 |
| Intubation | 2 (3.5) | 3 (5.3) | 0.64 |
| CVD | 0.68 | ||
| Yes | 18 (31.7) | 16 (28.1) | |
| No | 39 (68.3) | 41 (71.9) | |
| DM | 0.82 | ||
| Yes | 14 (24.6) | 13 (22.8) | |
| No | 43 (75.4) | 44 (77.2) | |
| HLP | 0.75 | ||
| Yes | 5 (8.8) | 6 (10.5) | |
| No | 52 (91.2) | 51 (89.5) | |
| CVA | 0.72 | ||
| Yes | 4 (7) | 5 (8.8) | |
| No | 53 (93) | 52 (91.2) |
a Values are expressed as mean SD or No. (%).
Among the participants, 43.9% had at least one underlying condition, such as cardiovascular diseases, diabetes mellitus, cerebrovascular accidents, or hyperlipidemia, while 56.1% had no prior medical history. The frequency of patients with a history of underlying diseases was 45.6% in the TCZ group and 42.1% in the standard treatment group, with no significant difference between the groups (P-value = 0.7). During hospitalization, 4.4% of the patients were intubated (Table 1).
Out of the patients included in this study, 41.8% died. The mortality rate was 47.4% (27 patients) in the TCZ group and 56.1% (32 patients) in the control group, with no statistically significant difference between the two groups (P-value = 0.34). Additionally, there was no significant difference in mortality rates between the two groups in patients under 60 years old (P-value = 0.8), with 13 deaths in the TCZ group and 14 in the control group. However, among patients aged 60 years and older, the mortality rate was significantly higher in the control group (19 deaths) than in the TCZ group (13 deaths). The chi-square test confirmed that this difference in mortality for patients over 60 years was statistically significant (P-value = 0.03) (Table 1).
In female patients, the mortality rate in the TCZ group was 44.1%, compared to 50% in the control group. Among male patients, mortality was 52.2% in the TCZ group and 62.1% in the control group. Although the mortality rates differed, no statistically significant relationship was found between gender and mortality in either group (P-value = 0.64 for females and P-value = 0.47 for males) (Table 1).
The mean duration of hospitalization was 20.16 ± 17.30 days for all patients. The average hospital stay was 20.72 ± 19.23 days in the TCZ group and 19.60 ± 15.29 days in the control group, with no statistically significant difference between the groups regarding the length of hospital stay (P-value = 0.84) (Table 2).
| Variable | Tocilizumab Administration | P-Value | |||
|---|---|---|---|---|---|
| No (n = 57) | Yes (n = 57) | ||||
| Mean ± SD | Median (Q1, Q3) | Mean ± SD | Median (Q1, Q3) | ||
| Hospitalization | 19.60 ± 15.29 | 14 (9, 28) | 20.72 ± 19.23 | 14 (9, 27) | 0.84 |
Abbreviations: SD, standard deviation; Q1, first quartile; Q3, third quartile.
5. Discussion
A comprehensive meta-analysis conducted by the WHO observed that administering TCZ in combination with corticosteroids resulted in a significant reduction in mortality rates among COVID-19 patients. Additionally, a 2021 study by Klopfenstein et al. conducted a meta-analysis of randomized trials focused on the use of TCZ. Their findings revealed a one-month mortality rate of 24.5% in the TCZ-treated group compared to 29.1% in the control group (14, 15). Notably, the use of TCZ was not associated with an increased risk of secondary infections or other adverse events. They attributed differences between the studies to factors such as disease severity, ICU admission, high-flow oxygen requirements, intubation, and mechanical ventilation. However, a study by Veiga et al. in 2019 cautioned that the use of TCZ should be carefully considered, as there was no significant difference in 28-day mortality in their findings (14-16).
In COVID-19 infections, patients requiring 10 liters (L) or more of oxygen were closely monitored. The decision to intubate early or late was made after ICU admission. Hypoxemia in patients needing more than 10 L of oxygen was assessed using the Spo2/Fio2 ratio, which correlates with the Pao2/Fio2 ratio in ARDS patients. Guidelines recommend using High-Flow Nasal Cannula (HFNC) or non-invasive ventilation (NIV) to treat ARDS or acute respiratory failure in COVID-19 patients, with intubation advised if no improvement occurs within 2 hours of using NIV or HFNC (17, 18). In our study, 4.5% of patients were intubated, with a lower rate in the TCZ group (3.5%) compared to the control group (5.3%), though this difference was not statistically significant. Various studies have explored the impact of TCZ on clinical outcomes and the need for intubation in COVID-19 patients. A study by Klopfenstein et al. (15) indicated that patients receiving TCZ required less intubation. Similarly, a systematic review by Kyriakopoulos et al. (17), which analyzed 52 studies on the effect of TCZ on COVID-19 patients, found that the mortality rate after TCZ administration decreased by 11% in clinical trials and by 31% in observational studies. Additionally, TCZ reduced the need for intubation by 19% (18-20).
Genetic changes and variants of the SARS-CoV-2 virus contribute to the complexity of COVID-19. The virus can undergo genetic alterations, leading to new variants with distinct characteristics, some of which may increase disease severity or confer resistance to existing drugs with each wave. Another important factor is the timing of drug administration, especially regarding IL-6 inhibitors, where timing may be critical (21, 22). Using these medications in the later stages of the disease may reduce their effectiveness. Additionally, the optimal timing and combination of these medications with other treatments can impact their efficacy. In our study, TCZ was initiated within 24 hours of admission for all patients to minimize the risk of bias. Since patients were in the ICU with severe conditions, we also provided standard treatment in addition to TCZ.
In trials conducted by Salama et al., and Hermine et al., beneficial outcomes were observed with TCZ in patients with severe COVID-19 undergoing intubation and invasive mechanical ventilation. However, these studies reported no statistically significant difference in overall mortality rates (22-24).
Pulmonary inflammation and severe lung damage in COVID-19 patients can trigger a cytokine storm, characterized by an abnormal systemic release of cytokines such as tumor necrosis factor α (TNF-α), interleukins (IL-1β, IL-2, IL-6), interferons (IFN-α, IFN-β, IFN-γ), and monocyte chemoattractant protein-1 (MCP-1) (25, 26). This excessive release of cytokines, also known as CRS, occurs as the immune system responds to the pathogenic invasion. Elevated levels of cytokines such as C-reactive protein (CRP), IL-6, and d-dimer are correlated with a higher risk of mortality. Consequently, targeting these cytokines has become a promising approach to mitigate the harmful inflammatory response (27-29).
Controlling the cytokine storm is crucial to improving the prognosis of patients with severe COVID-19. Some studies suggest that IL-6 inhibitors may be particularly effective in older patients or those showing elevated inflammatory markers like ferritin and C-reactive protein (30, 31). In our study, we observed a significantly higher mortality rate in elderly patients in the control group compared to those receiving TCZ. The lower mortality in the TCZ group may be attributed to the positive effects of the drug. Research has indicated that TCZ reduces mortality in older adults with severe COVID-19. Geriatric patients experience physiological changes associated with aging, which affect their ability to mount effective immune responses, particularly after the age of 40 or 50 (32, 33). This diminished immune response increases the vulnerability of the elderly to emerging infections like COVID-19. Additionally, underlying conditions such as cardiovascular, musculoskeletal, and metabolic diseases, as well as malignancies, increase the likelihood of poor clinical outcomes in elderly COVID-19 patients (32-34).
Aging is also associated with an increased inflammatory response, which can exacerbate the effects of severe infections and cytokine storms (14-16). COVID-19 has been described as a condition that can trigger a cytokine storm, with systemic cytokine levels in severe cases reaching levels similar to those observed in other critical infections like ARDS. In contrast, local inflammation is more common in mild and moderate cases. Thus, TCZ plays an important role in preventing disease progression and organ dysfunction in patients with severe COVID-19 (21, 22).
The strength of our study lies in its broad scope, focusing on patients with severe COVID-19 requiring ICU hospitalization. The patient selection process was thorough, targeting individuals who met the specific criteria for TCZ administration, guided by clinical and inflammatory markers during the pandemic. It is essential to acknowledge that extended hospital stays, the need for rehabilitation, and higher mortality rates are typical among patients with severe COVID-19. However, one limitation of our study is its retrospective design, which may introduce selection bias. Additionally, the single-center nature of the study might restrict the generalizability of the findings to other populations or settings.
5.1. Conclusions
Our research demonstrated the effects of TCZ in treating severe COVID-19 cases, particularly focusing on patients with CRS. Notably, administering TCZ to patients aged 60 and above was associated with reduced mortality rates. These findings highlight the need for further studies to investigate the safety, efficacy, and optimal treatment duration of TCZ in severe COVID-19 cases.