1. Background
Thalassemia is the most common genetic disorder in the world, passed from parents to children. In this disease, due to a defect in the hemoglobin gene (the protein that carries oxygen in the blood), the number of red blood cells is significantly reduced, and oxygen does not adequately reach the entire body. It exists in three forms: Minor, intermediate, and major (1).
A common clinical problem in patients with thalassemia major who are treated with blood transfusions is iron overload and toxicity, which can lead to the destruction of the heart muscle, liver, or endocrine glands. People with thalassemia accumulate iron in the heart muscle, leading to damage and eventual paralysis. However, if this complication is diagnosed in time, its effects can be prevented. For this reason, annual check-ups are recommended for these patients (2-4).
Unfortunately, despite the reversibility of iron overload cardiomyopathy, the late appearance of liver involvement symptoms and echocardiographic findings often results in a poor prognosis for patients (5, 6). For this reason, early diagnosis of iron overload in the heart and liver is essential for proper treatment, improving complications, and increasing patient survival (7, 8).
Common methods for assessing iron load in thalassemia patients include total iron-binding capacity (TIBC), serum iron, serum ferritin, liver biopsy, echocardiography, and T2* magnetic resonance imaging (MRI) (9-12). Serum ferritin level is a proven marker related to iron accumulation in organs, but its level may be influenced by inflammatory conditions, infectious diseases, and malignancy (13). Electrocardiography (ECG) provides valuable insights into cardiac involvement and complications related to iron overload in patients with thalassemia major (14). The standard, non-invasive gradient echo T2* MRI is used to determine the amount of tissue iron, and R2* MRI relaxometry of the liver has shown a good correlation with hepatic iron content (HIC) (15, 16).
Therefore, it is very important to evaluate the adequacy of treatment in these patients using proven diagnostic methods (17, 18). The main approach and innovation of this study is to examine the electrocardiographic findings of patients with dominant thalassemia and evaluate them alongside advanced MRI and laboratory information that have not been analyzed together previously.
2. Objectives
In this context, we aimed to investigate the status of heart and liver iron overload using MRI in thalassemia patients and to determine the relationship between T2* and R2* measurements with serum ferritin levels, aspartate aminotransferase / alanine aminotransferase (AST/ALT) enzyme ratio, and heart function. The objective is to assess whether understanding the relationship between these indicators can ensure the adequacy of treatment. The findings of this study, while clarifying the important diagnostic aspects of this multifaceted disorder, will have significant clinical and research implications for the future.
3. Methods
This study is a descriptive epidemiological and analytical study based on hospital and clinic data. A total of 108 beta-thalassemia major patients, whose medical history was recorded at Taleghani Hospital in Abadan, were selected, and the required information was extracted.
3.1. Inclusion Criteria
Beta-thalassemia major patients who underwent regular blood transfusions during the one-year study period. Patients with clinical signs and symptoms of heart disease, impaired renal or liver function, or infectious diseases were excluded.
The desired information was collected from the patients' medical records, and written consent was obtained from all patients included in this research.
3.2. The Evaluated Parameters
The serum ferritin level was determined using an immunoassay analyzer (AIA-360, India) and measured in ng/mL. The ALT and AST activity was measured using an automatic analyzer device (HITACHI 902, Japan) and reported in mg/dL. T2 map of the liver: * The sequence used was a multi-echo gradient echo with T2* weighting.
Imaging Parameters: TR = 120 ms, TE number = 8 - 16 echoes, TE first = 1 - 2 ms, TE final = 14 - 18 ms, FA = 15 - 25.
3.3. Number of Slices
Three to four slices with a thickness of 10 mm and an appropriate distance to ensure the entire liver is covered. The interval of changes between echo times was optionally set by the operator.
The tool used was ROI, which, when placed in the desired location, provides the values of T2*, R2*, and the amount of iron, along with the T2* decay exponential graph. The obtained images were loaded into specialized software to extract the required numbers. The data from this study were processed using STAR MAP (General Electric Company) and MAPIT (Siemens). Images were evaluated by three independent reviewers who were blinded to other patient information.
3.4. Imaging Parameters
TR = 3/1 ms, TE number = 8 - 16 echoes, TE first = 1 - 2 ms, TE final = 14 - 18 ms, FA = 15 - 25. The change interval between echo times was optionally set by the operator. Number of slices: Three slices with a thickness of 8 mm. After obtaining appropriate local images and 4-chamber, 2-chamber views, 3 incisions were made in the basal part, middle part of the ventricle, and apex of the heart. The desired numbers were extracted, and the images were uploaded into special software. The data of this study were processed using Cardiac VX (General Electric Company) and Myo Maps (Siemens).
The purpose of cardiac post-processing is to extract the desired numbers from the left myocardium. For this purpose, the myocardium should be contoured using the available tools. By using ROI and placing it in the desired location, T2*, R2*, and iron content were determined. In all patients, echocardiography was performed after receiving the packed cells.
Portable two-dimensional (2D) echocardiography (MC-PL-6018P, China) was performed using a Vivid 7 Dimension (GE Healthcare, USA) with a 2.5 or 3.5 MHz phased array transducer. Echocardiographic assessment was carried out by an expert cardiologist.
Data analysis was performed using statistical software SPSS version 26. Chi-square tests and t-tests were used to evaluate the differences in qualitative and quantitative variables, respectively. The sample size was determined using a formula to compare independent values. The Shapiro-Wilk test was performed to assess the normality of the data. Pearson correlation analysis was conducted to determine the relationship between different variables, including serum ferritin levels, cardiac parameters [T2_cardiac, R2_cardiac, ejection fraction (EF), tricuspid regurgitation gradient (TRG), pulmonary arterial pressure (PAP), tricuspid annular plane systolic excursion (TAPSE)], and HIC.
4. Results
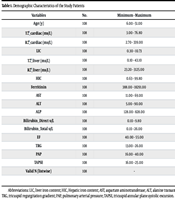
In this study, 108 (45/63 F/M) thalassemia major patients with an average age of (21.4 ± 9.3) were investigated. Table 1 indicates the demographic characteristics, and the averages of the other variables were examined.
| Variables | No. | Minimum - Maximum | Mean ± SD |
|---|---|---|---|
| Age (y) | 108 | 6.00 - 51.00 | 21.4537 ± 9.30117 |
| T2⃰_cardiac (ms/L) | 108 | 3.00 - 76.80 | 25.9769 ± 15.43925 |
| R2⃰_cardiac (ms/L) | 108 | 2.70 - 339.00 | 64.2556 ± 58.63964 |
| LIC | 108 | 0.30 - 10.73 | 1.9965 ± 1.91766 |
| T2⃰_liver (ms/L) | 108 | 0.10 - 43.10 | 3.3750 ± 6.02514 |
| R2⃰_liver (ms/L) | 108 | 23.20 - 3125.00 | 889.1704 ± 778.49160 |
| HIC | 108 | 0.63 - 99.80 | 28.3432 ± 24.29913 |
| Ferritinin | 108 | 388.00 - 18291.00 | 3893.2130 ± 3320.33650 |
| AST | 108 | 11.00 - 69.00 | 31.0056 ± 12.24713 |
| ALT | 108 | 5.00 - 90.00 | 39.5204 ± 17.44695 |
| ALP | 108 | 128.00 - 828.00 | 318.5741 ± 85.46281 |
| Bilirubin_direct u/L | 108 | 0.10 - 9.80 | 0.9648 ± 1.23109 |
| Bilirubin_total u/L | 108 | 0.10 - 26.00 | 1.8860 ± 2.95840 |
| EF | 108 | 40.00 - 55.00 | 49.7222 ± 4.96875 |
| TRG | 108 | 13.00 - 26.00 | 17.2870 ± 3.77659 |
| PAP | 108 | 16.00 - 40.00 | 21.5278 ± 5.74937 |
| TAPSE | 108 | 16.00 - 25.00 | 19.5926 ± 1.75636 |
| Valid N (listwise) | 108 | - | - |
Demographic Characteristics of the Study Patients
Among the studied patients, the reported liver iron levels were as follows: Normal in 2 (1.9%), mild iron overload in 12 (11.1%), moderate iron overload in 10 (9.3%), severe iron overload in 13 (12%), and very severe liver iron overload in 71 patients (65.7%) (Table 2). Likewise, the distribution of iron overload levels in the heart showed that most patients had normal cardiac overload (54.6%), while others had mild (13.0%), severe (22.2%), moderate (9.3%), and very severe (0.9%) overload, respectively (Table 3).
| Valid | Frequency (%) | Valid Percent | Cumulative Percent |
|---|---|---|---|
| Mild | 12 (11.1) | 11.1 | 11.1 |
| Moderate | 10 (9.3) | 9.3 | 20.4 |
| Normal | 2 (1.9) | 1.9 | 22.2 |
| Severe | 13 (12.0) | 12.0 | 34.3 |
| Very severe | 71 (65.7) | 65.7 | 100.0 |
| Total | 108 (100.0) | 100.0 | - |
Hepatic T2⃰ Magnetic Resonance Imaging Categorizations
| Valid | Frequency (%) | Valid Percent | Cumulative Percent |
|---|---|---|---|
| Mild | 14 (13.0) | 13.0 | 13.0 |
| Moderate | 10 (9.3) | 9.3 | 22.2 |
| Normal | 59 (54.6) | 54.6 | 76.9 |
| Severe | 24 (22.2) | 22.2 | 99.1 |
| Very severe | 1 (0.9) | 0.9 | 100.0 |
| Total | 108 (100.0) | 100.0 | - |
Heart T2⃰ Magnetic Resonance Imaging Categorizations
Pearson correlation analysis was performed to determine the relationship between different variables, including serum ferritin levels, cardiac and liver parameters, and HIC. The analysis showed that there was no statistically significant relationship between T2_cardiac levels and ferritin in all categories of iron overload. Similarly, no statistically significant relationship was observed between R2_cardiac and ferritin levels in all categories of iron overload.
Additionally, in this study, no significant relationship between ferritin levels and cardiac parameters (EF, TRG, PAP, and TAPSE) was observed at any level of iron overload (P > 0.05).
However, when assessing the relationship between ferritin levels and iron deposition in the liver and heart without stratifying the levels of iron overload, a significant inverse linear correlation was found between serum ferritin levels and MRI T2*_liver (correlation coefficient = -0.98; P < 0.05).
In addition, a statistically significant direct correlation was found between ferritin levels and R2* liver (correlation coefficient = -0.76; P < 0.05) (Table 4).
| Variables | T2*-Liver | R2*-Liver |
|---|---|---|
| Ferritin (ng/mL) | -0.98 | -0.76 |
| P-value | 0.035 | 0.046 |
Correlation Between Serum Ferritin Levels and Hepatic and Cardiac Hemosiderosis Measures
Our study confirmed this, despite not observing a significant relationship between ferritin levels and different cardiac and hepatic parameters in different overload categories. When comparing the variables without considering the classification of iron overload groups, a significant inverse relationship was found between ferritin and T2* liver, and a weak direct relationship was shown between ferritin and R2* liver.
5. Discussion
Since the liver is the first organ to store iron in the body, most of the patients in our study also had severe iron overload in the liver. Based on MRI T2* measurements, 22.2% and 65.7% of the examined patients had severe iron overload in the heart and liver, respectively. There was no statistically significant correlation between serum ferritin levels and the reported cardiac parameters in any of the categories of iron overload (mild, moderate, normal, severe, and very severe). This unexpected lack of correlation challenges common assumptions about a direct relationship between serum ferritin and cardiac complications in thalassemia. While serum ferritin is a widely used biomarker for assessing iron overload, our findings suggest that it may not serve as a reliable indicator for diagnosing cardiac dysfunction in this patient population. Consistent with our study, Anderson et al. also failed to find a significant correlation (19). On the other hand, Yuksel et al. found that serum ferritin levels were significantly associated with T2* values of the liver, but not significantly associated with T2* values of the heart (20). The existence of these contradictions requires the re-evaluation of current diagnostic protocols and the development of monitoring approaches to reduce cardiac complications in thalassemia major patients. Based on these findings, annual T2* MRI is necessary because serum ferritin levels do not necessarily indicate heart iron levels.
These findings indicate a complex relationship between serum ferritin levels and cardiac function and emphasize the need for further research to elucidate the exact mechanisms involved in cardiac dysfunction and iron overload. Therefore, MRI T2* can be a useful method to evaluate heart and liver iron in patients prone to iron storage in a shorter period of time. The possibility of an inverse interaction between serum ferritin and hepatic iron deposition emphasizes the complexities of iron distribution in individuals with thalassemia. This issue, in turn, can have consequences for treatment decisions and the evaluation of the effectiveness of liver iron chelation treatments.
Previous studies have reported that cardiac and hepatic iron overload detected by MRI T2* correlates with serum ferritin levels in thalassemia patients, although the results are conflicting (16, 21-23). One of the important factors that may affect the divergence of findings between this study and previous research is the sample size. Smaller sample sizes may limit the study's power to detect subtle relationships between variables by introducing statistical heterogeneity. In contrast, larger studies have the advantage of collecting more representative data from a wider population, potentially providing results that are generalizable to a broader patient population. Hence, differences in findings may be partially attributable to variations in sample size and limitations. However, the interesting finding of an inverse relationship between serum ferritin levels and iron deposition in the liver raises questions about the potential impact of therapeutic interventions. It is conceivable that patients with higher serum ferritin levels in this study may have received earlier and more aggressive treatments with chelating agents designed to reduce iron overload. These interventions can effectively prevent excessive deposition of iron in vital organs such as the liver, leading to an inverse correlation between ferritin levels and organ iron content. These findings emphasize the importance of considering history and treatment intensity as important variables in iron metabolism studies. As disorders related to iron deposition progress, a more precise understanding of how therapeutic interventions interact with biomarkers such as serum ferritin will be essential to improve patient care and outcomes. In conclusion, the present study elucidates the complex relationship between serum ferritin levels, cardiac parameters, and hepatic iron deposition in patients with thalassemia major. The lack of a significant association between serum ferritin and cardiac parameters intensifies the need to develop more comprehensive cardiac assessment strategies. The inverse relationship identified between serum ferritin and hepatic iron deposition emphasizes the complexity of iron distribution in thalassemic individuals and points to a re-evaluation of iron monitoring methods. These findings have profound clinical implications and pave the way for further exploration of accurate assessment and management of iron overload in thalassemia major patients.