1. Background
Being the second leading cause of mortality worldwide and a significant public health concern, cancer is expected to account for 1.9 million new cases by the end of 2021, based on the American Cancer Society (1). Conventional treatment methods for cancer include surgery, chemotherapy, radiation therapy, targeted therapy, immunotherapy, and hormone therapy (2, 3). While cytotoxicity and cytostasis can result from chemotherapy and radiation treatment (4), they are frequently associated with severe adverse effects and a significant possibility of recurrence. The most common side effects include tiredness, gastrointestinal and skin issues, neuropathies, hair loss, and bone marrow suppression (5). Multimodal and synergistic cancer therapies that enhance patient outcomes are crucial.
With the use of nanotechnology, tumors can be treated and imaged more effectively by using nano-vehicles that can deliver one or more therapeutic cargos together with contrast agents. For improved medication administration, phototherapy, immunization, immunotherapy, and imaging, nanoparticles (NPs) can be "smart designed" (6, 7). It is possible to create NPs with a variety of physicochemical and surface characteristics that may be adjusted to improve molecular and cellular delivery, lengthen circulation durations, facilitate NP passage through biological barriers, and regulate cargo release (7, 8). To address the challenges faced by cancer therapy, NPs can also be engineered to incorporate multiple therapeutic modalities within a single system (6).
Recently, efforts have been made to employ nanoparticles to address the limitations of current medical procedures. Nanoparticle-based drug delivery systems have shown promise in cancer management and treatment due to their favorable pharmacokinetics, precise targeting, reduced side effects, and decreased drug resistance (9, 10). As nanotechnology has advanced, several nano-therapeutic drugs have been extensively marketed and commercialized, with many more reaching clinical stages since 2010. Nanoparticle-based drug delivery systems continue to demonstrate potential in improving cancer treatment outcomes (11). For instance, curcumin nanoparticles have shown the capability to overcome Docetaxel resistance in castration-resistant prostate cancer (12). Chitosan nanoparticles exhibit cytotoxic effects against human breast cancer cell lines (MDA-MB-231, SK BR3) (13). In bladder cancer cells, gold nanoparticles increase the expression of pro-apoptotic genes BAX and CASPASE3 while reducing VEGF and BCL2 gene expression, which are associated with angiogenesis and anti-apoptotic processes (14). Similarly, silver nanoparticles have demonstrated anticancer activity in the MCF-7 human breast cancer cell line (15).
Fibrin, a naturally occurring protein produced during the blood coagulation cascade, is widely used in surgical procedures for hemostasis and wound healing (16, 17). Due to its exceptional biocompatibility and biodegradability, fibrin serves as a promising matrix for stem cell differentiation and tissue regeneration (18, 19). It has been utilized as a scaffold for cell growth and differentiation and as an effective delivery vehicle for the sustained release of drugs and proteins (18-20). When carboplatin was embedded in fibrin, it remained lethal to retinoblastoma cells, suggesting fibrin's potential as a carrier for anti-cancer drugs (21).
2. Objectives
This study aimed to evaluate the effects of fibrin nanoparticles (FNPs) on breast cancer cells, contributing to the exploration of FNPs as a potential therapeutic strategy.
3. Methods
3.1. Cell Line and Cell Culture
A human breast epithelial cell line (MCF-7 cells) was obtained from Iran Cell Bank, Pasteur Institute of Iran, Tehran, I. R. Iran. The cells were cultured in DMEM/F12 and maintained in an incubator at 37°C with 95% humidity and 5% carbon dioxide. Cells were passaged using Trypsin/EDTA (Biotech, Germany, NO100-780). Cells were left untreated or treated with 100 µg/mL FNPs for 5 days, which were prepared in our previous study (22-24). The cells were grown in an incubator at 37°C with 5% CO2 in air.
3.2. RT-PCR Method
Total RNA from MCF-7 cells was extracted using the FavorPrep™ blood/cultured cell total RNA Purification Mini Kit (FAVORGENE, FABRK001). Reverse transcription was performed using the Thermo Scientific RevertAid First Strand cDNA Synthesis Kit, with BR441 cat number (BIOFACT). To detect gene expression, PCR Master Mix SYBR™ Green BIOFACT High ROX was utilized. Triplicate reactions were conducted. The primers (Metabion) for the qRT-PCR experiment are listed in Table 1. Real-time PCR and data collection were performed using an ABI Applied Biosystems StepOne Plus real-time PCR detection system. GAPDH expression was used to normalize gene expression (Table 1).
| Gene (Human) | Primer (5' - 3') | Product Length (bp) |
|---|---|---|
| BCL2 | 105 | |
| Forward | GATGGGATCGTTGCCTTATGC | |
| Reverse | CAGTCTACTTCCTCTGTGATGTTGT | |
| VEGF | 103 bp | |
| Forward | CCTTGCCTTGCTGCTCTACC | |
| Reverse | ATCCATGAACTTCACCACTTCGT | |
| GAPDH | 127 bp | |
| Forward | GCTCATTTCCTGGTATGACAACG | |
| Reverse | CTCTCTTCCTCTTGTGCTCTTG |
DNA Primers
The cells (1 × 105) were seeded, cultured on the slide, and treated for 5 days. After treatment, the cells were washed with PBS and fixed with 4% paraformaldehyde (Sigma, L2020) for 15 minutes at room temperature. The cells were then permeabilized for one hour at 37°C using 0.2% Triton X-100 (Sigma, T8787). Following permeabilization, the cells were rinsed with PBS and treated with goat serum for 45 minutes. Subsequently, the cells were incubated overnight at 4°C with a primary antibody (mouse monoclonal Anti-BCL2, PDM o16, Cell Marque, USA) at a dilution of 1: 500, followed by three PBS washes.
Next, the cells were treated with a secondary antibody (goat-anti-Mouse polyclonal secondary antibody, AK CMG356, Cell Marque, USA) at a 1:500 concentration for one hour at 37°C. After three additional PBS washes, they were stained with 3,3'-diaminobenzidine (DAB Chromogen, 945D-41, Cell Marque, USA) at a concentration of 3 ng/mL for ten minutes at room temperature. Apoptotic cells were examined under an Olympus light microscope in Hamburg, Germany, and five distinct microscopic fields were analyzed for each sample. The percentage levels of BCL2 and VEGF protein were estimated based on a threshold using ImageJ version 1.52 h.
3.3. Data Analysis
Each test was conducted in triplicate, and results are presented as mean ± SEM. A one-sample t-test was used to evaluate statistical differences between the control and treated groups. SPSS software was utilized for statistical analysis, and graphs were generated using Prism 8 software. The significance level for all tests was set at P < 0.05.
4. Results
4.1. The Expression of Genes
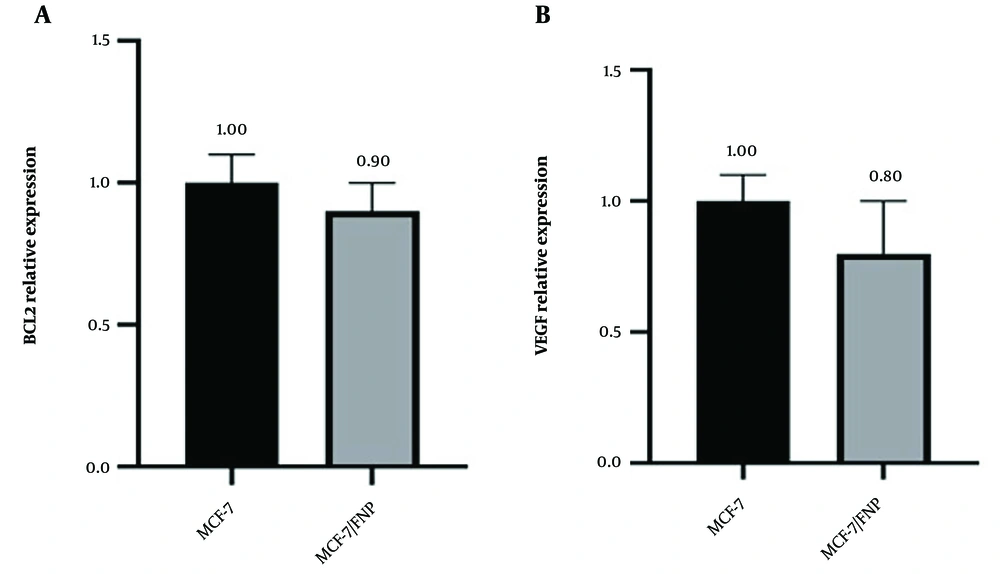
To elucidate the cellular mechanisms underlying the effects of nanoparticles, we assessed whether FNP treatment alters gene expression in cancer cells, specifically BCL2 and VEGF. BCL2 expression was decreased in the MCF-7/FNP-treated groups compared with the control group. Similarly, VEGF expression was reduced in the MCF-7/FNP group (Figure 1).
4.2. Immunocytochemistry
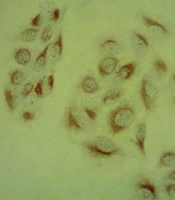
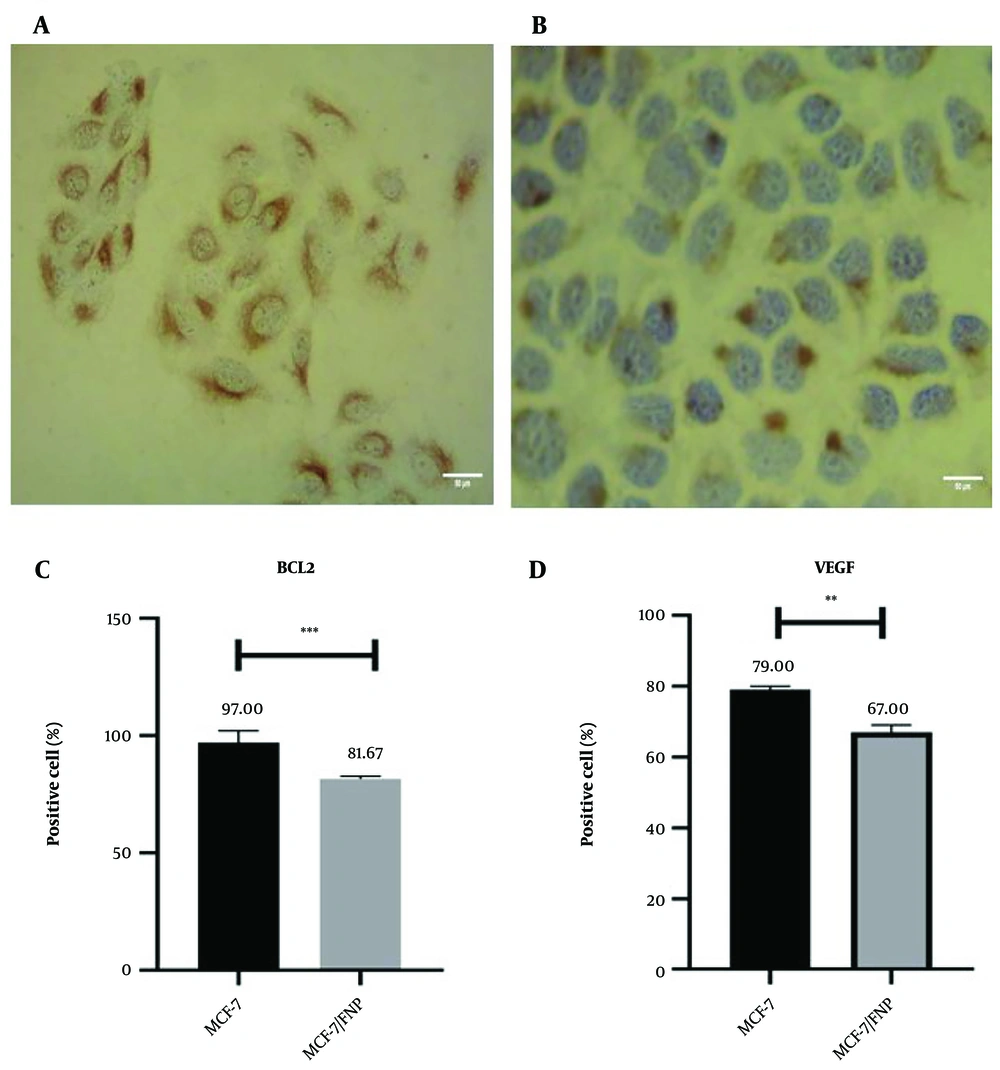
In Figure 2A and B, the black arrows indicate the nucleus, while the red arrows highlight protein expression in the cytoplasm of cancer cells. Cell count results using ImageJ software showed a significant reduction in BCL2-positive cells in the FNP-treated groups compared to the control group (MCF-7) (Figure 2C) (P < 0.001). Additionally, the VEGF-positive cells in the treated group were significantly decreased compared to the control (P < 0.01) (Figure 2D).
Investigation of the effect of fibrin nanoparticle (FNP) BCL2 and VEGF protein expression in MCF-7/FNP group. The black arrow shows the nucleus and the red arrow shows the protein expression in the cytoplasm of cancer cells. Examining the expression of proteins in different groups in 3-dimensional conditions. In this test, ** P < 0.01, *** P < 0.001. Error bar represents standard deviation.
5. Discussion
We discovered that FNP exhibited strong antitumor activity by inducing apoptosis. The results from gene expression and immunocytochemistry analyses demonstrated that FNP prompted apoptosis in cancer cells, affecting the expression of relevant genes. Nanotherapeutics for cancer are evolving rapidly, addressing limitations of conventional drug delivery methods, such as non-specific biodistribution, poor targeting, low water solubility, and limited oral bioavailability (10). This study employed fibrin nanoparticles to evaluate their effects on breast cancer cell lines.
Our investigation revealed a decrease in gene expression related to cancer proliferation. Daei et al. reported similar apoptotic and anti-angiogenic effects of gold nanoparticles on human bladder cancer (5637) cells, showing a decrease in the mRNA expression of anti-apoptotic BCL2 and angiogenic VEGF genes, while pro-apoptotic genes like BAX and CASPASE3 were upregulated (14). Likewise, Dolati et al. demonstrated the anticancer potential of nanoparticles on MCF-7 and SKBR3 breast cancer cells. Their findings indicated that anti-apoptotic and angiogenic genes BCL2 and VEGF were significantly downregulated, while pro-apoptotic genes, including BAX and CASP3, were notably increased (25).
Similarly, hydroxyapatite nanoparticles have shown promising results in prostate cancer cells (RM1), leading to a significant downregulation of BCL2 and VEGF while increasing BAX and CASPASE3 mRNA expression levels (26). These findings align with our study, where immunocytochemistry confirmed reduced protein expression of BCL2 and VEGF in MCF-7 cells treated with FNPs, reinforcing FNPs' potential to activate apoptotic pathways in cancer cells.
Our results further support the findings of Liang et al., who observed downregulation of BCL2 and VEGF in prostate cancer cells treated with hydroxyapatite nanoparticles (26). Similarly, Qiu et al. demonstrated that gold nanoparticles reduced the expression of VEGF and BCL2 proteins in glioblastoma cells (27). Our findings suggest that FNPs can induce apoptosis in cancer cells by downregulating anti-apoptotic and angiogenic factors, thereby activating cell death pathways.
5.1. Conclusions
Our findings indicate that FNP exhibits strong antitumor properties, primarily by downregulating anti-apoptotic genes. This study suggests that FNP may activate the intrinsic apoptotic pathway, leading to cell death in MCF-7 cancer cells through the reduction of VEGF and BCL2 levels. While the current results are promising, further in vivo research is necessary to confirm the protective benefits of FNP against breast cancer and to fully understand its therapeutic potential.