1. Background
Toxoplasmosis is one of the most common parasitic zoonoses worldwide, affecting both humans and animals (1). The definitive hosts of Toxoplasma gondii are felines, which play a crucial role in the parasite’s life cycle. Oocysts, a resistant form of the parasite, are found in the feces of infected cats (2). These oocysts require 1 to 5 days in the soil to become infectious and can remain viable for up to 18 months, depending on environmental factors (3). Humans and other warm-blooded animals serve as intermediate hosts in the parasite’s life cycle (4). In these hosts, T. gondii reproduces asexually. Initially, the parasite’s tachyzoites multiply rapidly within various host cells. Subsequently, tissue cysts containing bradyzoites are formed. The life cycle of T. gondii in the intermediate host concludes with the development of tissue cysts, where bradyzoites persist and replicate slowly throughout the host’s lifetime. These tissue cysts are infectious to both definitive and other intermediate hosts. When ingested by felines, bradyzoites undergo asexual replication in the epithelial cells of the small intestine, followed by sexual reproduction. Oocysts are then released into the intestinal lumen and excreted in the feces, thereby contaminating the environment (1).
Infection with T. gondii often occurs through the ingestion of various infectious forms of the parasite. Oocysts can contaminate food and water sources, representing a route of transmission. Additionally, the consumption of raw or undercooked meat containing tissue cysts is another source of infection (5). Tachyzoites have also been identified in the milk of several intermediate hosts, suggesting that consuming contaminated unpasteurized milk may pose a transmission risk (6). Toxoplasmosis can also be transmitted through blood transfusion and organ transplantation (7, 8). Congenital transmission may occur when a pregnant mother acquires acute toxoplasmosis, allowing the parasite to cross the placenta and infect the fetus. Vertical transmission is also possible due to the reactivation of latent infection, particularly in immunocompromised pregnant women (9-11).
The seroprevalence of T. gondii worldwide ranges from 30% to 60%, with at least one-third of the global population estimated to be infected (12). Global seroprevalence shows significant variation and may exceed this range in endemic areas. For instance, in African populations, seroprevalence rates have been reported as high as 90% (13). A meta-analysis conducted in Iran estimated the seroprevalence of toxoplasmosis in the general population at 39.3%. This study also showed that in the northern areas of Iran, particularly around the Caspian Sea, where the climate is humid, infection is very common (54%). In contrast, colder mountainous areas such as Ardabil province and warm regions like Sistan and Baluchistan province reported lower seroprevalence rates of 18.3% and 22.8%, respectively (14). In another study, the seroprevalence of T. gondii in the general population of Abadan city was estimated at 43.95% (15).
The strength of the host immune system is a critical factor in the course of infection with *T. gondii*. In immunocompetent individuals, the infection is usually asymptomatic and remains latent (16). Tissue cysts are more common in the nervous system, ocular tissues, and skeletal muscles, though they may also form in visceral organs such as the liver. These cysts can persist without triggering a host immune response (17). In contrast, toxoplasmosis is an opportunistic infection in immunocompromised patients, often presenting as encephalitis, myocarditis, and pneumonitis. Reactivation of latent toxoplasmosis due to cyst rupture in such patients can be life-threatening (18). Cancer patients are particularly vulnerable to severe toxoplasmosis due to both disease-related immunosuppression and the immunosuppressive effects of anticancer treatments, which can reactivate latent T. gondii infection (19). Furthermore, the overall prevalence of T. gondii infection is higher among cancer patients compared to the general population, and its clinical implications in this group underscore the need for investigation (20).
2. Objectives
Given the limited number of studies conducted on the prevalence of toxoplasmosis among immunocompromised patients both globally and in the study area, further research is warranted. Therefore, the present study aimed to investigate the seroprevalence of T. gondii and related risk factors in cancer patients compared to a control group among patients attending teaching hospitals in Abadan and Khorramshahr, Iran.
3. Methods
3.1. Study Area
Abadan and Khorramshahr cities are located in Khuzestan province, in the southwest of Iran. Abadan is bordered by the Arvand River to the west and the Bahmanshir River to the east, and it is separated from Khorramshahr by a branch of the Karun River. These two cities are situated on the border between Iran and Iraq, approximately 53 kilometers from the Persian Gulf. The region is characterized by a desert climate. There is a noticeable difference in air temperature between summer and winter. During the summer, temperatures can exceed 50°C, while winter temperatures usually range between 16°C and 20°C. Frequent dust storms occur annually. This region experiences extremely hot and dry summers, contrasted by mild and wet winters (21, 22).
3.2. Study Population
This study included two groups: Cancer patients and a control group. The cancer patient group consisted of 128 individuals diagnosed with various types of cancer who visited Taleghani, Shahid Beheshti, and Valiasr hospitals in Abadan and Khorramshahr cities in 2021 for treatment or diagnostic procedures. A questionnaire containing demographic information was provided to each participant, and written informed consent was obtained following a full explanation of the study procedures. Cancer diagnoses were confirmed by reviewing medical documents and records under the supervision of an oncologist. The control group included 128 individuals with no history of cancer, selected from outpatients attending the same hospitals. After obtaining written informed consent, these participants also completed the demographic questionnaire. The absence of cancer or other chronic underlying diseases was confirmed through detailed medical history and examination of their medical records. The case and control groups were matched for age and gender.
3.3. Serology
Approximately 5 mL of venous blood was collected from qualified participants in both the patient and control groups. Blood samples were transferred to tubes without anticoagulant and centrifuged at 3500 rpm for 10 minutes. The separated serum was kept at -20°C until analysis. Serological testing was performed to detect specific anti-T. gondii IgG and IgM antibodies using a commercial kit. All samples were tested under the same conditions. Both IgG and IgM antibodies were measured in all individuals from the case and control groups (23, 24).
3.4. Questionnaire
Each participant was asked to complete a structured questionnaire containing the following questions: Age; gender (male/female); cancer type (non-hematologic malignancy/hematologic malignancy); education level (university/high school diploma or less); place of residence (urban/rural); source of drinking water (purified/unpurified); history of consuming raw or undercooked meat (yes/no); washing and disinfection of vegetables (yes/no); owning a pet (yes/no); history of contact with cats (yes/no); and exposure to soil (yes/no).
3.5. Data Analysis
Collected laboratory results and questionnaires were analyzed using SPSS software version 19. Descriptive statistics, including frequency and percentage, were used. A P-value of less than 0.05 was considered statistically significant. No sensitivity or interaction analyses were performed due to the cross-sectional nature and primary objective of the study.
4. Results
This study included 128 cancer patients and 128 control subjects without underlying diseases, all of whom were referred to teaching hospitals in Abadan and Khorramshahr. The overall seroprevalence of anti-T. gondii antibodies was 41.4% (53/128) in the cancer patient group and 49.2% (63/128) in the control group. The seroprevalence of anti-T. gondii IgG was 39.84% (51/128) in cancer patients and 46.88% (60/128) in the control group, with no significant difference (P = 0.313). The prevalence of anti-T. gondii IgM antibodies was 2.34% (3/128) in cancer patients and 3.13% (4/128) in the control group, also showing no statistically significant difference (P = 1.0). One participant from each group (cancer and control) was simultaneously positive for T. gondii IgG and IgM antibodies.
In this study, the age range of cancer patients was 18 - 75 years, while the control group participants ranged from 17 - 70 years. Among cancer patients, the single individual under 20 years was seropositive for T. gondii. Seroprevalence rates in other age groups were as follows: Over 60 years, 43.6%; 41 - 60 years, 41.3%; and 21 - 40 years, 25%. However, these differences were not statistically significant (P = 0.497). In the control group, the highest seroprevalence was observed in individuals over 60 years (64.3%), followed by 21 - 40 years (51.9%), 41 - 60 years (46.5%), and 0% in those under 20 years, with no statistically significant difference among age groups (P = 0.463). Comparison between the two groups also demonstrated no significant relationship between age and seropositivity for T. gondii (P ≥ 0.05).
In the cancer group, the seroprevalence was 42.6% (40/94) in males and 38.2% (13/34) in females, showing no significant difference (P = 0.814). In the control group, 39.8% (37/93) of men and 74.3% (26/35) of women were seropositive for T. gondii; the seroprevalence was significantly higher in females than in males (P = 0.001). Furthermore, the seroprevalence of T. gondii in females in the control group was significantly higher than in females in the cancer group (P = 0.005).
In the cancer patient group, 41.3% (50/121) of urban residents and 42.9% (3/7) of rural residents were seropositive for T. gondii, with no statistically significant difference between the two groups (P = 1.000). In the control group, rural residents showed a significantly higher seroprevalence (83.3%; 10/12) compared to urban residents (45.7%; 53/116) (P = 0.029).
The seroprevalence of T. gondii infection in cancer patients who consumed purified water was 41.3% (50/121), and the seroprevalence in patients who consumed unfiltered water was 42.9% (3/7), with no statistically significant difference (P = 1.000). In the control group, the seroprevalence in individuals who consumed untreated water (83.3%, 10/12) was significantly higher than in those who had access to purified drinking water (45.7%; 53/116) (P = 0.029). No significant difference in seroprevalence of T. gondii was observed between the cancer patients and control groups in terms of drinking water (P ≥ 0.05).
Among cancer patients, 50 (39.1%) had hematologic malignancies, while 78 (60.9%) had various solid tumors. The seroprevalence of T. gondii infection was 43.6% (34/78) in patients with solid tumors and 38% (19/50) in patients with hematological malignancies; however, this difference was not statistically significant (P = 0.658).
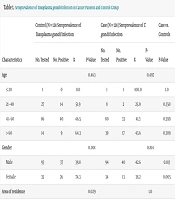
Furthermore, no significant associations were found between T. gondii seroprevalence and risk factors including education level, consumption of raw or undercooked meat, washing and disinfection of vegetables, contact with soil, contact with cats, and pet ownership in the patient or control groups. More details of these variables are provided in Table 1.
| Characteristics | Control (N = 128 Seroprevalence of Toxoplasma gondii Infection | Case (N = 128) Seroprevalence of T. gondii Infection | Case vs. Controls | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. Tested | No. Positive | % | P-Value | No. Tested | No. Positive | % | P-Value | P-Value | |
| Age | 0.463 | 0.497 | |||||||
| ≤ 20 | 1 | 0 | 0.0 | 1 | 1 | 100.0 | 1.0 | ||
| 21 - 40 | 27 | 14 | 51.9 | 8 | 2 | 25.0 | 0.350 | ||
| 41 - 60 | 86 | 40 | 46.5 | 80 | 33 | 41.3 | 0.599 | ||
| > 60 | 14 | 9 | 64.3 | 39 | 17 | 43.6 | 0.309 | ||
| Gender | 0.001 | 0.814 | |||||||
| Male | 93 | 37 | 39.8 | 94 | 40 | 42.6 | 0.813 | ||
| Female | 35 | 26 | 74.3 | 34 | 13 | 38.2 | 0.005 | ||
| Area of residence | 0.029 | 1.0 | |||||||
| Urban | 116 | 53 | 45.7 | 121 | 50 | 41.3 | 0.584 | ||
| Rural | 12 | 10 | 83.3 | 7 | 3 | 42.9 | 0.187 | ||
| Education level | 1.0 | 0.841 | |||||||
| High school diploma or less | 89 | 44 | 49.4 | 104 | 44 | 42.3 | 0.397 | ||
| University | 39 | 19 | 48.7 | 24 | 9 | 37.5 | 0.542 | ||
| Drinking water | 0.029 | 1.0 | |||||||
| Purified | 116 | 53 | 45.7 | 121 | 50 | 41.3 | 0.584 | ||
| Unpurified | 12 | 10 | 83.3 | 7 | 3 | 42.9 | 0.187 | ||
| Raw meat consumption | 0.616 | 0.804 | |||||||
| Yes | 81 | 38 | 46.9 | 72 | 31 | 43.1 | 0.752 | ||
| No | 47 | 25 | 53.2 | 56 | 22 | 39.3 | 0.225 | ||
| Disinfection of vegetables | 0.941 | 0.193 | |||||||
| Yes | 109 | 53 | 48.6 | 111 | 43 | 38.7 | 0.179 | ||
| No | 19 | 10 | 52.6 | 17 | 10 | 58.8 | 0.970 | ||
| Contact with cat | 0.183 | 0.998 | |||||||
| Yes | 81 | 44 | 54.3 | 93 | 38 | 40.9 | 0.105 | ||
| No | 47 | 19 | 40.4 | 35 | 15 | 42.9 | 1.0 | ||
| Pet keeping | 0.744 | 0.833 | |||||||
| Yes | 52 | 27 | 51.9 | 63 | 25 | 39.7 | 0.261 | ||
| No | 76 | 36 | 47.7 | 65 | 28 | 43.1 | 0.734 | ||
| Contact with soil | 0.347 | 0.486 | |||||||
| Yes | 29 | 17 | 58.6 | 31 | 15 | 48.4 | 0.593 | ||
| No | 99 | 46 | 46.5 | 97 | 38 | 39.2 | 0.375 | ||
| Cancer type | - | 0.658 | |||||||
| Hematologic malignancy | 0 | 0 | 0.0 | 50 | 19 | 38.0 | - | ||
| Solid tumor | 0 | 0 | 0.0 | 78 | 34 | 43.6 | - | ||
| Antibodies | - | - | |||||||
| IgG | 128 | 60 | 46.88 | 128 | 51 | 39.84 | 0.313 | ||
| IgM | 128 | 4 | 3.13 | 128 | 3 | 2.34 | 1.0 | ||
5. Discussion
Acute infection or reactivation of latent T. gondii infection can cause life-threatening complications such as encephalitis, particularly in immunocompromised individuals (5). Cancer patients represent a vulnerable population due to their suppressed immune system resulting from the malignancy and from chemotherapy, immunosuppressive drugs, and long-term hospitalizations (25). Consequently, cancer patients need special attention, as they are at risk for developing severe complications from toxoplasmosis.
The seroprevalence of anti-T. gondii IgG in the cancer patient group of the present study is similar to findings from studies conducted in cancer patients in Ahvaz (45.2%) (26) and Tabriz (40.66%) (27). Additionally, the seroprevalence of anti-T. gondii antibodies in the control group (49.2%) was higher than the reported average in the general Iranian population (39.3%) (14). In a study conducted by Cong et al. in China (28), the seroprevalence of anti-T. gondii IgG was 35.56% in cancer patients and 17.44% in the control group, both markedly lower than the rates observed in our study. Moreover, their study reported a significantly higher seroprevalence in cancer patients compared to the control group, which is not consistent with our study. In another study conducted in Saudi Arabia in cancer patients (29), the seroprevalence rates of anti-T. gondii IgG and IgM were 29.9% and 0.7%, respectively, which are lower than those observed in the present study. The seroprevalence of anti-T. gondii IgM in cancer patients of the present study was lower than that reported in the investigations by Ghasemian et al. (26), Khayat and Ghareh (27), and Cong et al. (28).
Variations in climate, nutrition, and public health levels may account for differences in T. gondii seroprevalence in studies conducted in different geographic regions. Also, the presence of stray animal populations and limited public access to properly purified water in the study area may contribute to the elevated number of seropositive cases observed. In general, the seroprevalence of toxoplasmosis tends to increase with age (30), likely due to an increased chance of exposure to various infectious forms of T. gondii over a lifetime. In the present study, only one cancer patient under the age of 20 tested seropositive for T. gondii. In other age groups, the seroprevalence of toxoplasmosis increased with age: 43.6% in those older than 60 years, 41.3% in the 41 - 60 years group, and 25% in the 21 - 40 years group. However, the differences in seroprevalence across these age groups were not statistically significant (P = 0.497). In the control group, the highest seroprevalence was observed in individuals over 60 years of age (64.3%), though this difference was also not statistically significant (P = 0.463).
Several studies on toxoplasmosis in cancer patients have reported that with increasing age, the seroprevalence of T. gondii also increases (26, 29, 31). In the present study, the unequal distribution of cancer patients across different age groups, especially the limited number of cases under 20 years old, which caused a high seroprevalence (100%), may have influenced the comparability of our findings with those of other studies. Additionally, differences in lifestyle, health misconceptions, and prolonged exposure to infectious agents may contribute to the higher seroprevalence observed in older patients. Gender may also indirectly affect the risk of toxoplasmosis through job roles and exposure to sources of infection in daily life.
In the present study, the seroprevalence of T. gondii among male cancer patients (42.6%) was higher than among female patients (38.2%), although this difference was not statistically significant. However, in the control group, the seroprevalence of T. gondii in females (74.3%) was significantly higher than in males (39.8%) (P = 0.001). Moreover, the difference between women in the patient group and women in the control group was also significant (P = 0.005).
Several studies on cancer patients have also reported no significant association between the seroprevalence of T. gondii and gender (26, 28, 31-33), which aligns with the results in cancer patients of this study. Most of these studies reported a higher seroprevalence of T. gondii in females than in males (26, 28, 31, 33), similar to our control group. In the study conducted by Mostafa et al. (34), a significantly higher seroprevalence was observed in females than in males. The lower number of female participants in our study may affect the results.
In the study area, women are often housewives who are more frequently involved in food preparation. They may have more contact with meat, fruit, and vegetables potentially contaminated with T. gondii, particularly if proper hand hygiene is not observed, which may justify the significantly higher seroprevalence observed among women in the control group. In rural areas, the occupation of most people is agriculture and animal husbandry, and hygiene standards are generally lower than in urban areas. These factors may contribute to an increased risk of toxoplasmosis.
In the present study, the seroprevalence of anti-T. gondii antibodies in cancer patients was higher in rural residents (42.9%) compared to urban residents (41.3%), but this difference was not statistically significant (P = 1.0). However, in the control group, rural residents exhibited a significantly higher seroprevalence of T. gondii compared to urban residents (83.3% vs. 45.7%, P = 0.029). A study conducted by Yu et al. (35) on patients with colorectal malignancies reported a higher seroprevalence in rural residents, and this difference was statistically significant in the patient group. Studies by Barazesh et al. (33) and Ali et al. (31) found higher seroprevalence of T. gondii in the urban population, although these differences were not statistically significant.
The higher seroprevalence rates in rural areas may be attributed to lower knowledge of disease prevention among residents and greater environmental exposure to T. gondii oocysts in soil that has been contaminated by infected cat feces. The majority of rural residents in the current study work in agriculture, which increases their exposure to contaminated soil. One of the largest outbreaks of symptomatic toxoplasmosis in Canada resulted from contamination of a municipal water supply with oocysts shed by mountain lions (36). Toxoplasma gondii oocysts can survive in cold water for up to 54 months and remain infective. While they are resistant to conventional drinking water chlorination, many modern municipal water treatment systems in developed countries can remove them from drinking water (37).
In the present study, the seroprevalence of T. gondii was lower among individuals who used purified drinking water compared to those without access to purified water. Although this difference was not statistically significant in the cancer patient group, it was significant in the control group (P = 0.029). Yu et al. (35) similarly reported a lower seroprevalence of T. gondii in people who consumed treated municipal water compared to those who obtained their drinking water from untreated sources like wells or rivers, though the difference was not statistically significant. Cong et al. (28) found no significant relationship between the source of drinking water consumed and the seroprevalence of T. gondii, in line with our results obtained in cancer patients.
The uncontrolled growth of stray cats and their prey species in the current study area enables T. gondii to complete its life cycle, resulting in the widespread environmental shedding of oocysts. The oocysts remain in the environment for long periods after contaminating water sources. The oocysts’ resistance to standard chlorination processes, combined with restricted access to properly purified drinking water in this region, makes contaminated water a primary transmission route. This environmental risk factor could explain why T. gondii seroprevalence is high in the study population.
In many hematological malignancies, a degree of cellular immunodeficiency is present. The immunosuppressive effects of chemotherapy and other treatment modalities make patients with these cancers highly susceptible to acute T. gondii infection or reactivation of latent toxoplasmosis (31). In the present study, the seroprevalence of T. gondii was higher among patients with solid tumors (43.6%) than in those with hematological malignancies (38.0%), although this difference was not statistically significant (P = 0.658). Abdel Malek et al. (19) observed a higher seroprevalence in solid tumor patients than in hematologic malignancy patients, although the difference was not statistically significant (P = 0.06). Conversely, in the study conducted by Ali et al. (31), the seroprevalence of anti-T. gondii IgG was significantly higher in patients with hematological malignancies compared to those with solid tumors, which is contrary to the findings of our study (P = 0.002).
The observed differences between studies can be explained by multiple factors, including variations in chemotherapy protocols, cancer stage at sampling time, and patient health and immune status. These variables affect susceptibility to opportunistic infections such as toxoplasmosis.
5.1. Conclusions
The results of this study show a high prevalence of T. gondii infection in Abadan and Khorramshahr. In the control group, living in rural areas, the consumption of untreated drinking water, and female gender were significantly associated with higher seroprevalence of infection. Although no statistically significant association was observed between cancer and the seroprevalence of anti-T. gondii antibodies, cancer patients face an elevated risk of toxoplasmosis complications. Therefore, it is necessary to consider toxoplasmosis as an opportunistic infection in the management and treatment of cancer patients. It is recommended to perform screening tests before prescribing immunosuppressive drugs in these patients.
5.2. Limitations
In this case-control study, the ELISA test was performed on collected sera. The lack of molecular-based techniques to confirm serological findings, which could have strengthened the diagnostic accuracy, is the main limitation of this research project.