1. Context
The identification of microbes is a crucial objective within the field of microbiology, as certain bacteria can significantly impact the health of humans and other animals. Additionally, some unidentified strains produce valuable metabolites with applications in pharmaceuticals, as well as in the food, cosmetic, and health sectors. Therefore, it is essential for microbiologists to accurately identify these strains and harness their potential. Conversely, some unidentified strains may be involved in diseases affecting humans or animals, and their identification can serve as a foundation for effective treatment strategies.
One of the most widely used molecular techniques is 16S rRNA sequencing, as it is present in all microorganisms and serves a consistent function across different species. Its sequence is highly conserved, comprising nearly 1500 bases, which facilitates straightforward sequencing. Although this approach has significantly advanced microbiology, it is inadequate for the precise identification of closely related bacterial groups (such as Streptococcus mitis and coagulase-negative staphylococci), which cannot be definitively differentiated by this method (1). Thus, it is beneficial to carry out biochemical tests to validate the identification of these particular types of bacteria.
Each bacterium is required to produce a specific collection of enzymes to facilitate its functions; therefore, these enzymes can serve as targets for the development of biochemical tests aimed at identifying the genera of specific bacteria. Various tests, such as catalase, oxidase, and urease, along with others mentioned in this review, can be employed to distinguish particular types of microorganisms (2-4). Biochemical techniques serve as dependable tools for the preliminary identification of bacteria by examining the enzymatic activities associated with each strain. Each strain shows a different reaction to tests based on its specific enzymatic characteristics, which causes changes in the pH of the culture medium and changes in the medium's color. In the following section, we will examine several biochemical tests employed for this purpose, detailing the procedures for conducting each test and the methods for interpreting the results.
2. Biochemical Tests
2.1. Catalase Test
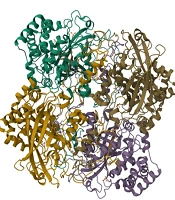
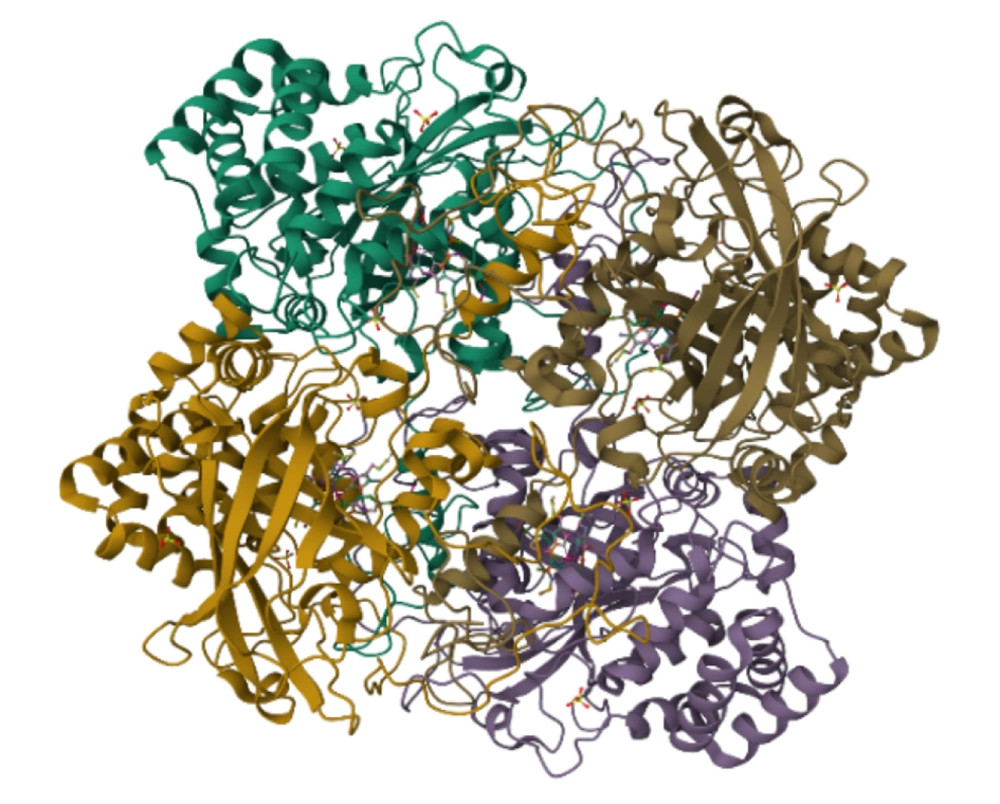
Numerous bacteria possess flavoproteins that can reduce oxygen (O2) to generate hydrogen peroxide (H2O2) or superoxide (-O2). These byproducts are extremely toxic and can damage cellular structures, necessitating that bacteria develop mechanisms to defend against harmful oxygen derivatives; failure to do so can result in their demise. To address this challenge, many bacteria produce enzymes that safeguard them from toxic oxygen species. Obligate aerobic, aerobic, and facultative anaerobic bacteria typically contain the enzyme superoxide dismutase, which decomposes superoxide into hydrogen peroxide. Subsequently, hydrogen peroxide is further degraded by the enzymes catalase or peroxidase (5-8)
Catalase is an enzyme composed of four polypeptide chains, with each chain consisting of over 500 amino acids. This enzyme contains four porphyrin groups that facilitate its reaction with hydrogen peroxide (9-11) (Figure 1). Anaerobic bacteria do not possess this enzyme, rendering them incapable of surviving in the presence of oxygen. The presence of catalase can be identified by applying a few drops of hydrogen peroxide onto the surface of a bacterial colony grown in a trypticase soy agar medium (7, 12, 13). The catalase test is an important diagnostic key for identifying and differentiating bacteria. For example, it can differentiate Streptococcus (catalase negative) from Staphylococcus and Micrococcus (catalase positive) (14).
2.1.1. Catalase Test Protocol
- The substrate used in this test is 3% hydrogen peroxide.
- With a wooden applicator, transfer some of the microbial samples from the center of a colony to the surface of a glass slide.
- Add a few drops of 3% hydrogen peroxide to it and mix.
- A positive catalase reaction is indicated by the production of oxygen gas bubbles on the surface of the slide (13).
2.1.2. Catalase Analyze
If the bacteria possess the catalase enzyme, the production of oxygen gas occurs as described in the aforementioned reactions. The presence of oxygen gas bubbles on the colony's surface signifies a positive catalase test (Figure 2), whereas the absence of gas production indicates a negative result.
To conduct the catalase test, it is essential to consider the following aspects: The hydrogen peroxide solution is prone to instability; therefore, it is advisable to utilize a freshly prepared solution for testing purposes. Additionally, employing a wooden applicator for colony retrieval is recommended, as the use of an iron loop may result in a false positive reaction. Since some bacteria have enzymes other than catalase that cause the decomposition of hydrogen peroxide, the formation of small bubbles in small numbers after 20 to 30 seconds does not indicate a positive reaction.
2.2. Oxidase Test
The oxidase test is another biochemical test that was originally developed for Neisseria bacteria and later used for the Enterobacteriaceae group. It is used to check for the presence of the enzyme cytochrome oxidase, which plays a crucial role in electron transfer during aerobic respiration. This test is a differential method for distinguishing species such as Pseudomonas, Neisseria, Moraxella, Vibrio, Campylobacter, Pasteurella, and Brucella. Among the gram-negative pathogenic bacteria, Neisseria and Pseudomonas are oxidase-positive, while the Enterobacteriaceae family is oxidase-negative, allowing for differentiation through this simple test (13, 15, 16).
Oxidase enzymes are integral to the electron transport system in aerobic respiration, where cytochrome oxidase uses oxygen as an electron acceptor (17). The ability of bacteria to produce the enzyme cytochrome oxidase can be determined by performing an oxidase test. Cytochrome oxidase can oxidize the substrate tetramethyl-para-phenylenediamine-dihydrochloride, forming the colored product indophenol. When a small amount of the organism producing this enzyme is applied to a piece of paper soaked in the substrate, a dark purple color is observed (Figure 3). This substrate acts as an electron donor in the presence of the oxidase enzyme and free oxygen, oxidizing to a black compound.
In this test, a 1% solution of tetramethyl-para-phenylenediamine-dihydrochloride is used. Typically, 50 mg of the powder is placed into small plastic lidded containers for daily use, with 5 mL of distilled water added, and one vial used each day. A clean filter paper is placed on the surface of a sterile plastic plate and moistened with a few drops of fresh reagent. A small amount of a young colony (less than 24 hours old) is removed using a clean wooden applicator and placed on the moistened filter paper. The color change to blue and purple within 10 to 20 seconds is observed, noting that the timing of the color check is crucial (13).
There are various methods for performing this test, including the filter paper method, direct method, swab method, oxidase test strip method, and tube method. One technique employed is the dry filter paper method, where Whatman 1 filter paper is soaked in a freshly prepared 1% solution of tetramethyl-p-phenylenediamine dihydrochloride. After approximately 30 seconds, the strips undergo freeze-drying and are preserved in a dark, airtight container with a screw cap. To conduct the test, a strip is extracted and positioned in a Petri dish or on a slide. The surface is dampened with distilled water, and the colony intended for testing is collected using a loupe or a glass or plastic Pasteur pipette and immersed in the moist region. It is important to avoid using metal instruments in this test, as they may produce a false positive outcome (18).
2.2.1. Oxidase Analyze
In this test, organisms that contain the enzyme oxidase, such as species of the genus Neisseria, change the color of the colony to purple within 10 seconds. Conversely, organisms that do not possess this enzyme, such as Escherichia coli, show no color alteration within the same timeframe. This reaction yields a positive result as a consequence of oxidation in the air; therefore, if the test results are evaluated after 20 to 30 seconds, it may yield a false positive outcome, rendering it diagnostically insignificant.
Bacteria that are classified as oxidase-negative can be anaerobic, aerobic, or facultative. A negative oxidase result indicates merely the absence of cytochrome c oxidase, which is responsible for oxidizing the reactant, while these organisms may utilize alternative oxidases for electron transfer during respiration (19).
A positive result from the dry filter paper method is indicated by the appearance of a dark purple color within 5 to 10 seconds. A 'delayed positive' result is recognized when a color change occurs between 10 and 60 seconds, whereas a negative result is indicated by the paper remaining colorless or changing color after more than 60 seconds (18).
2.3. Urease Test
Buffered broth (Rustigian & Stuart urea broth) or solid Christensen's urea agar are used for this purpose (20). Besides facilitating the detection of various Enterobacteriaceae, this test is instrumental in identifying and distinguishing particular species of Brucella, assisting in the identification of encapsulated yeasts, and providing supplementary support in the identification of specific types of gram-negative coccobacilli.
Since urea cannot be sterilized by autoclaving, first prepare the urea base culture medium and sterilize it by autoclaving. Then, sterilize the urea using a filter and add it to the culture medium. To perform the test, a small amount of bacteria is first grown on a solid medium as a surface and a loop inside the liquid medium, and then placed at 37 degrees Celsius for 18 - 24 hours (13, 20).
2.3.1. Urease Analyze
Urea is classified as a diamide, and certain bacteria possessing the enzyme urease are capable of utilizing urea to generate ammonia, carbon dioxide, and water. The ammonia produced is subsequently transformed into ammonium carbonate along with other substances, increasing the pH level of the culture and rendering it alkaline. A positive reaction is indicated by the entire liquid medium and the surface of the solid medium turning pink, whereas a negative reaction is characterized by the medium remaining yellow (Figure 4).
This medium is referred to as phenol red, which exhibits an orange color at a pH of 6.8 and transitions to pink and purple at a pH of 8.1 (13). In the urease test process, there is no significant difference between liquid and solid culture media. Both types contain a phenol red reagent that changes color with pH variations.
2.4. SIM Test
SIM medium is a semi-solid medium utilized for the vertical and deep cultivation of bacteria. This medium is effective in demonstrating bacterial motility. To inoculate the organism, a pure culture aged between 18 to 24 hours is utilized on appropriate media, including KIA and SIM. Using an inoculation needle, pierce the center of the medium to a depth of 1 cm. The medium must then be incubated at 37°C for a duration of 24 to 48 hours. If no motility is observed, the medium should be stored at room temperature, between 21 to 25°C, for a period of 7 days.
The incubation temperature is crucial, as many motile organisms exhibit motility at temperatures ranging from 15 to 25°C, while they may be nonmotile at their optimal growth temperature of 37°C. If there is a suspicion that the organism may be motile at a lower temperature, it is advisable to inoculate two tubes simultaneously, maintaining one at 37°C and the other at 21 to 25°C (21).
2.4.1. SIM Analyze
If the bacteria are motile (positive motility), in addition to growth in the inoculation line, the environment around this line also shows clear growth or turbidity (Figure 5). The SIM medium is a semi-solid culture medium that allows for the vertical and deep growth of bacteria. This medium not only showcases bacterial motility and indole production but also facilitates the detection of hydrogen sulfide (H2S) production.
Bacteria utilize several sulfur-containing compounds, such as peptone, methionine, cysteine, and sodium thiosulfate, leading to the generation of H2S. When H2S interacts with ferric ions, it results in the formation of black ferrous sulfide (13).
2.5. Hydrogen Sulfide Production Test
The media used to investigate the production of H2S include bismuth sulfide, citrate sulfide agar, lysine iron agar (LIA), KIA, triple sugar iron (TSI), and SIM. Additionally, H2S production can be quantified using XLD, HE, and SS media, as well as lead acetate strips. It is important to highlight that SIM media exhibits greater sensitivity in detecting H2S compared to KIA and TSI media.
In the SIM culture medium, bacteria metabolize several sulfur-containing compounds such as peptone, methionine, cysteine, and sodium thiosulfate, leading to the production of H2S, which subsequently reacts with ferric ions to form black ferrous sulfide (13, 22).
2.5.1. Hydrogen Sulfide Analyze
After culturing bacteria in the desired medium, observing a black color in the culture medium indicates the production of H2S gas.
2.6. Triple Sugar Iron Test
The TSI test serves to identify members of the Enterobacteriaceae family and to distinguish them from other gram-negative enteric bacilli. The TSI culture medium contains 1% lactose, 1% sucrose, and 0.1% glucose. The varying concentrations of these sugars are utilized to assess the organisms' ability to ferment them. Additionally, phenol red functions as a pH indicator, reflecting changes in the medium's acidity due to carbohydrate fermentation (23).
2.6.1. Triple Sugar Iron Test Protocol
- First, write the bacteria characteristics on the tubes containing the TSI medium.
- Under sterile conditions, using a sharp-pointed needle, culture the depth of the tube in a vertical line and the inclined surface in a zigzag pattern.
- Keep at 37°C for 18 to 24 hours.
- Following the incubation period, assess the tubes for different chemical reactions (23).
2.6.2. Triple Sugar Iron Analyze
The process of acid production through sugar fermentation is indicated by a color change from red to yellow. This medium also contains sodium thiosulfate and iron sulfate. Sodium thiosulfate serves as the substrate for the production of H2S, and iron sulfate acts as a color reagent, forming a colored precipitate in the presence of H2S (23).
2.6.3. Interpretation of Triple Sugar Iron Test Results
- The red slope indicates an alkaline environment, while the yellow depth of the tube signifies an acidic condition. In this scenario, only glucose undergoes fermentation, resulting in the production of minimal acid, which alters the medium's color to yellow. Given the low concentration of glucose, it is rapidly consumed, prompting the bacteria to utilize alternative compounds within the medium for their energy requirements. In instances where the bacteria cannot ferment lactose, they resort to utilizing peptone compounds, leading to the generation of ammonium ions. This process results in the culture medium becoming alkaline, thereby changing the color of the slope to red. However, due to the limited oxygen availability in the deeper section of the tube, bacterial growth is slow, causing this area to remain yellow (24, 25).
- Yellow slope (acidic) and yellow tube depth (acidic): This condition indicates the fermentation of lactose or sucrose, and due to the high concentration of these two sugars and the production of large amounts of acid, the entire medium turns yellow (24, 25).
- Red slope (alkaline) and red tube depth (alkaline): This condition indicates that none of the sugars in the medium are fermented, and the production of acid, gas, and H2S gas is negative. Instead, the peptones are consumed under aerobic or anaerobic conditions, producing ammonia, which causes the medium to become alkaline (23-25).
- The occurrence of breaking, cracking, or rising of the culture medium inside the tube: This condition indicates the production of gas following sugar fermentation. Bacteria that produce acid and gas from sugar fermentation acidify the medium (yellow color), which in turn leads to the cracking or rising of the medium (24, 25) (Figure 6).
- Blackening of the culture medium: Some bacteria reduce sodium thiosulfate to sulfite and H2S gas, and H2S gas in the presence of iron sulfate creates a black precipitate of iron sulfide (23-25).
2.7. IMViC Tests
The four tests mentioned are collectively called IMViC (the first letter I is for indole, the first letter M is for methyl red, the first letter V is for Voges-Proskauer, and the first letter C is for citrate, with the letter i included for ease of expression of the set of abbreviations). The test outcomes vary depending on the type of bacteria; for example, in Escherichia coli, the results are typically – – + +, and in Klebsiella, they are + + – – (26).
2.7.1. Simon Citrate Test
Certain bacteria possess the capability to derive energy from alternative sources aside from carbohydrate fermentation. Sodium citrate, a salt derived from citric acid, serves as a nutrient or carbon source for these bacteria. The medium employed for the detection of citrate is known as Simmons Citrate agar. Bacteria that produce the enzymes citratease, citrase, and citrate lyase can utilize citrate effectively (27).
To conduct the test, a small quantity of bacteria is initially cultured on this medium as a surface layer and subsequently incubated at a temperature ranging from 35 to 37 degrees Celsius for 48 hours (26).
2.7.1.1. Simon Citrate Analyze
The reagent for this medium is bromothymol blue, which turns green at neutral pH, yellow at acidic pH (less than 6), and blue at alkaline pH (above 6.7). If the bacteria grow and produce a blue color, the reaction is considered positive (26) (Figure 7).
2.7.2. Methyl Red Test
Some bacteria are capable of fermenting glucose and producing acid. The resulting acid is identified by introducing a substance such as methyl red.
2.7.2.1. Method
(1) First, inoculate a few colonies of pure bacterial culture in a tube containing 2.5 mL of methyl red (MR)/voges-proskauer (VP) Broth.
(2) Incubate for 48 - 72 hours (maximum 5 days) at 37°C.
(3) Then add 5 drops of methyl red reagent to the broth and check the reaction result based on the resulting color change.
- The formation of yellow color is a negative reaction.
- The formation of red color is a positive reaction as a result of high acid production and pH < 4.5.
- The formation of orange color is a negative reaction.
(4) To shorten the conclusion time, about 0.5 mL of MR/VP Broth can be poured into a tube and a relatively large amount of microbes can be inoculated in it. After incubation for 18 - 24 hours at 37°C, add 1 - 2 drops of methyl red reagent to it and observe the reaction (26, 28).
2.7.2.2. Methyl Red Analyze
Methyl red serves as a pH indicator, exhibiting a color change from yellow at pH 6 to red at pH 4.4. Its capacity to indicate acidity is notably lower than that of other biological reagents, functioning effectively only in environments with high acidity, particularly during the fermentation of various acids derived from glucose. Certain Enterobacteriaceae generate acid primarily during the initial phase of incubation; however, only those bacteria capable of sustaining a low pH in the medium over an extended incubation period of 48 to 72 hours cause a positive result. Escherichia coli is used as a positive control and Enterobacter aerogenes is used as a negative control in this test (26, 28).
2.7.3. Voges-Proskauer Test
This reaction shows the formation of acetyl methyl carbinol or acetoin from glucose in the medium. Bacterial fermentation of glucose typically occurs through two primary pathways: The first is mixed acid fermentation, which yields a significant quantity of acid alongside a minor amount of ethanol; the second pathway is butylene glycol fermentation, characterized by the generation of small amounts of various acids while producing substantial quantities of butylene glycol and ethanol.
To perform this test, the bacteria are cultured in MR-VP medium (broth containing glucose and phosphate) and placed at a temperature of 35 - 37°C for 24 - 48 hours (the time is shortened if a concentrated culture is used) (23, 26, 28).
2.7.3.1. Voges-Prosqueur Analyze
After the incubation period, 15 drops of alpha-naphthol alkali solution (5% solution in 95% ethanol) and 10 drops of potash (40% in water) are added to each milliliter of the culture medium. After mixing, it is placed in a 37°C incubator for 15 to 30 minutes until a pink-red color appears in the medium, which indicates a positive reaction. A pale yellow color indicates a negative reaction. Klebsiella, Enterobacter, and Serratia react positively in this test (23, 26, 28-30).
Before introducing the reagents, it is essential to agitate the medium to ensure an adequate supply of O2. Also, add alpha-naphthol first, then potassium and creatine. The inclusion of creatine enhances the availability of guanidine, resulting in the development of a red color within 10 to 15 minutes. In this test, Enterobacter aerogenes serves as the positive control, while Escherichia coli functions as the negative control (23, 26, 28).
2.7.4. Indole Test
Some bacteria of the Enterobacteriaceae family, such as Escherichia coli, which contain the enzyme tryptophanase, can produce indole gas from the amino acid tryptophan (31, 32). To test for indole gas production, the bacteria are cultured in a medium containing tryptophan (1%) — tryptone broth is recommended — and incubated at 37°C for 24 hours. Then, a few drops of Kovac's reagent or Ehrlich-Boehme's reagent are added to the surface of the culture tube. If indole gas is present, a red ring forms on the surface of the medium 2 minutes after adding the reagent (26, 33).
2.7.4.1. Indole Analyze
If a color change occurs to yellow or turns reddish-brown, this reaction is negative. Media such as SIM, ornithine-motility-tryptophan (OMT), and indole nitrate are used to test for indole production. The culture medium used for the indole test must be free of sugar (glucose), as this sugar prevents the production of indole gas. Additionally, the availability of oxygen influences indole production; thus, facultative anaerobes tend to generate more indole in aerobic environments. Escherichia coli serves as the positive control, while Klebsiellapneumoniae acts as the negative control in this assay (26, 33).
3. Conclusions
There are various techniques available for bacterial identification. However, the use of widely accepted biochemical tests is preferred due to their ability to facilitate work processes, optimize time, and reduce costs associated with strain identification. By implementing biochemical tests and utilizing specific media along with characteristics like strain morphology, it is possible to identify unknown bacteria in the sample. Molecular technology predominantly constrains the use of biochemical tests; however, these tests remain in use within laboratories owing to the financial and time-consuming aspects of molecular technologies. Biochemical tests can be employed to identify species in the separation of microbial samples resulting from the growth of bacterial strains, along with a limited number of molecular identifications as required (34).
This article explored various biochemical tests utilized to identify gram-positive and gram-negative bacteria. In biochemical assays, it is common to document variations in time and temperature, particularly when significant factors such as inoculum size, test medium volume, or the type of container utilized must be considered. The outcomes of certain tests may be signified by the detection of acidity or alkalinity, frequently indicated by a color change resulting from a pH indicator incorporated within the test medium (13).
Different factors can disrupt laboratory tests, leading to pre-analysis errors that occur before the sample is assessed. Biochemical tests have certain limitations that, if not properly addressed, can cause testing errors. It is imperative to ensure that the bacterial samples are obtained fresh from the culture. In the case of certain assays like catalase and oxidase, employing a metal loop can produce misleading outcomes. In experiments that generate black sulfide gas, identifying the color of the culture medium at deeper levels proves to be challenging. It is important to note that the reagents employed can be unstable and should be freshly prepared for each test. The timing of the test readings is crucial; exceeding the recommended time may lead to incorrect results as the reagents can change color over time. Moreover, it is vital to consider both aerobic and anaerobic conditions for every strain and test.
Additionally, it is important to highlight that many bacteria pose challenges for identification through biochemical methods. Therefore, it is advisable to employ more precise techniques, such as 16S rRNA analysis, to accurately identify these organisms and ascertain their group and genus.