1. Context
Sepsis, the body’s extreme response to infection, is the third leading cause of death due to infection and has been increasing in recent years (1). This disease is the most common cause of death in the intensive care unit (ICU) (2), with the increase in the elderly population, the emergence of pathogens with extensive drug resistance (3), and the indiscriminate administration of antibiotics being the main causes of the rising prevalence of sepsis in recent years (3). Sepsis is accompanied by a severe inflammatory response to infection, characterized by the release of inflammatory cytokines (4) and severe oxidative stress (5). Clinically, sepsis is associated with a severe drop in blood pressure and excessive activity of vasoconstrictor agents, leading to the failure of vital body organs (6, 7). Despite advances in understanding the pathophysiology of this disease and the introduction of strong antimicrobial drugs, not much success has been achieved in reducing sepsis mortality.
A decrease in neutrophil counts (neutropenia, < 1.0 × 109/L) is associated with a weakened immune system in dealing with bacterial infections, which is common in sepsis (8). Granulocyte-colony stimulating factor (G-CSF) is one of the factors that stimulate the production of neutrophils by the bone marrow (9), and it seems that it can be effective in improving the immune capacity to fight infections (10). For this reason, the administration of G-CSF (filgrastim) has attracted the attention of researchers for strengthening the immune system and improving the production of neutrophils in septic patients.
For example, Aktas et al. (2015) showed that administration of rhG-CSF to sepsis infants was associated with increased neutrophil counts but had no effect on short-term mortality (11). Similarly, in another study, administration of GM-CSF, although associated with the improvement of neutropenia, had no effect in reducing sepsis or short-term mortality (12). Recently, Vacheron et al. showed that administration of 125 μg/m2 GM-CSF for 5 days to septic and septic shock patients had no effect on the prevention of ICU-acquired infections (13).
2. Objectives
Therefore, there are inconsistencies in the effectiveness of G-CSF in improving the clinical outcomes of sepsis patients. To address this knowledge gap, this meta-analysis was conducted to assess the impacts of G-CSF in the management of sepsis and septic shock in ICU-hospitalized patients.
3. Methods
A comprehensive electronic search strategy was employed to identify relevant studies. Major bibliographic databases, including PubMed and EMBASE, were systematically searched. A refined search strategy was developed utilizing a combination of medical subject headings (MeSH) terms and relevant keywords pertaining to sepsis, septic shock, G-CSF, the ICU setting, and randomized controlled trials (RCTs). Boolean operators (AND, OR, NOT) were strategically incorporated to ensure the precise retrieval of eligible studies. Additionally, manual searches of reference lists from retrieved articles and relevant conference proceedings were conducted to identify potentially pertinent studies not captured through the electronic database search.
3.1. Study Selection
A two-stage selection process employing rigorous criteria was implemented to guarantee the inclusion of high-quality RCTs in this meta-analysis. Two independent reviewers meticulously screened all retrieved citations based on titles and abstracts. Studies deemed potentially relevant based on predefined inclusion and exclusion criteria were subjected to a full-text assessment using a standardized eligibility form. This standardized form detailed study design, population characteristics, intervention details, and reported outcomes. Any discrepancies arising between reviewers during either stage of the selection process were resolved through discussion and, if necessary, by involving a third reviewer to achieve consensus. It is worth mentioning that the selection and review of the papers were carried out in September 2024 and lasted for one month.
3.2. Quality Assessment of Studies
The quality of each included randomized controlled trial (RCT) was assessed using the Cochrane collaboration’s tool for assessing risk of bias (RoB 2.0). This validated tool evaluates potential biases arising from several key domains, including randomization sequence generation, which assesses the method used to allocate participants to intervention groups, ensuring a fair chance of receiving G-CSF or control.
3.3. Inclusion Criteria
The inclusion criteria were as follows:
- Investigates the efficacy and/or safety of G-CSF (filgrastim) compared to placebo or standard care in patients diagnosed with sepsis or septic shock while admitted to an ICU setting.
- Employs a RCT design to ensure robust causal inferences and minimize the risk of bias.
- Reports at least one of the following clinically relevant outcomes:
- Mortality: Evaluates overall patient survival throughout the study period (all-cause mortality) with a specific focus on the critical first 28 days after enrollment (28-day mortality).
- Organ dysfunction: Assesses the impact of G-CSF on organ function using validated scoring systems such as sequential organ failure assessment (SOFA) or multiple organ dysfunction score (MODS). This provides a comprehensive picture of potential benefits beyond just mortality.
- Respiratory recovery: Investigates the duration of mechanical ventilation dependence, a marker of respiratory recovery and potential treatment effectiveness.
- Intensive care unit and hospital length of stay: Evaluates the time patients spend in the ICU and overall hospital stay. This can reflect treatment effectiveness, resource utilization, and potential discharge readiness.
3.4. Exclusion Criteria
The exclusion criteria were as follows:
- Language restriction: Studies published only in languages other than English were excluded due to potential translation biases and resource constraints.
- Pediatric populations: Studies involving patients under 18 years old were excluded due to potential physiological and disease course differences compared to adults.
- Prophylactic use of granulocyte-colony stimulating factor: Studies investigating the use of G-CSF to prevent sepsis rather than treat established cases were not considered.
- Incomplete data: Studies with missing or unavailable data for predefined outcomes were excluded to ensure reliable meta-analysis and avoid introducing bias.
3.5. Data Extraction and Quality Assessment
To minimize bias and ensure data accuracy, a standardized data extraction form was developed. Two independent reviewers collected relevant information from each included RCT.
3.6. Outcomes
The outcomes were patients’ mortality rate, SOFA scores, APACHE II scores, and ICU stay.
3.7. Statistical Analysis
Meta-analysis was performed using RevMan V.5.1 software. Continuous variables were used for data analysis. To check the degree of heterogeneity between studies, the I2 Index and chi-squared test were used. A random effects model was used for I2 > 50% and P < 0.1. Otherwise, a fixed effects model was used for data analysis.
4. Results
4.1. Study Selection
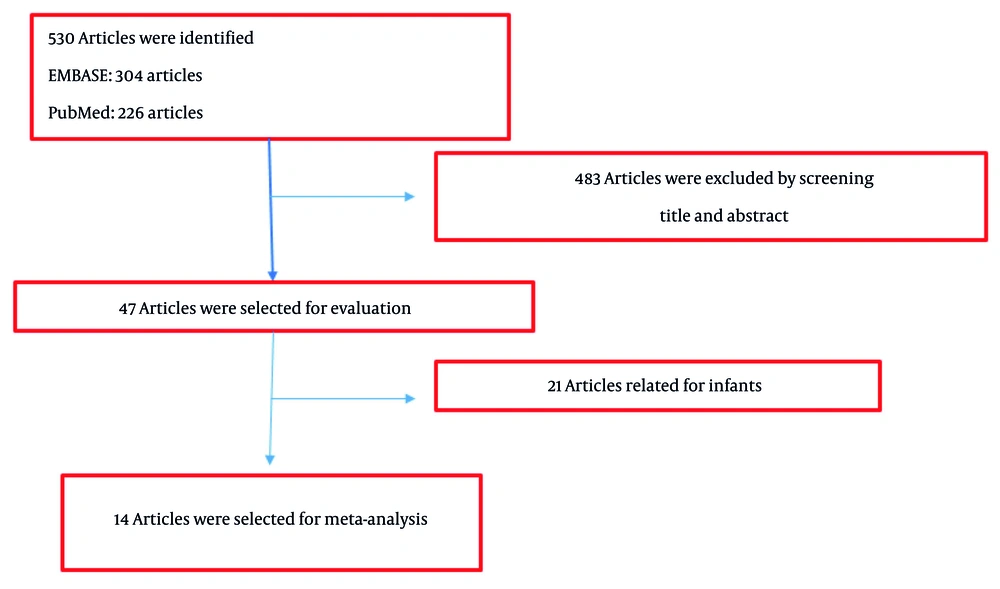
Relevant research was searched in two databases, EMBASE and PubMed, using appropriate keywords. A total of 304 articles were identified from EMBASE and 226 articles from PubMed. The abstracts and titles of these 530 articles were reviewed, and 47 articles were selected for a comprehensive review of the full text by reviewers. According to Figure 1, 14 articles were finally selected for conducting the meta-analysis (13-27). The characteristics of the selected studies are shown in Table 1.
| Authors, Year, Ref. | Study Design | Dose/Drug | Age | Main Findings |
|---|---|---|---|---|
| Cheng et al. (2007) (15) | RCT | 263 μg/day - lenograstim | 49 - 61 | The drug failed to affect mortality rate of severe sepsis patients. |
| Ishikawa et al. (2000) (16) | RCT | 2 μg/kg - lenograstim | 51 - 54 | The drug improved inflammatory responses in septic patients. |
| Meisel et al. (2009) (17) | RCT | 4 µg/kg - GM-CSF | 63 - 64 | GM-CSF administration for sepsis patients resulted in shorten ICU stay length and mechanical ventilation. |
| Presneill et al. (2002) (18) | Randomized phase II trial | 3 µg/kg - rGM-CSF | 45.6 - 62 | rGM-CSF did not affect mortality rate of severe sepsis patients; rGM-CSF activated neutrophils. |
| Root et al. (2003) (19) | RCT | 300 µg/day -filgrastim | 58.9 - 60 | Filgrastim failed to affect mortality rate of severe sepsis patients. |
| Rosenbloom et al. (2005) (20) | RCT | 3µg/kg/d - sargramostim | 56 - 63 | Sargramostim improved microbial resolution of infection without affecting vital organ function. |
| Schefold et al. (2010) (21) | RCT | 4 µg/kg - GM-CSF | 62 - 63 | GM-CSF improved anti-bacterial defense by decreasing IDO activity and catabolites related to the kynurenine pathway. |
| Stephens et al. (2002) (23) | RCT | 300 µg - G-CSF | - | G-CSF reduced mortality rate of septic shock patients and recommended as adjunctive therapy for septic shock. |
| Stephens et al. (2008) (22) | RCT | 263 µg/day - lenograstim | 48.9 - 51.0 | G-CSF failed to affect the outcomes of septic shock patients. |
| Tanaka et al. (2001) (24) | RCT | 2 µg/kg - lenograstim | 49 - 54.8 | Lenograstim improved inflammatory responses without affecting lung function. |
| Vacheron et al. (2023) (13) | RCT | 125 μg/m2 - sargramostim | - | Sargramostim failed toa affect sepsis patients’ outcomes including mortality and ICU-acquired infection. |
| Weiss et al. (1996) (25) | RCT | 1 µg/kg/day - filgrastim | - | Filgrastim improved neutrophils function and reduced inflammatory responses. |
| Weiss et al. (2003) (26) | RCT | 1 µg/kg/day - filgrastim | 63 - 64 | Filgrastim increased neutrophil counts and CD64 expression. |
| Wunderink et al. (2001) (27) | RCT | 300 µg - filgrastim | 49 - 56 | Filgrastim administration to septic shock and sepsis patients was safe. |
The Characteristics of Studies Used in Current Meta-Analysis
4.2. Meta-Analysis of Outcomes
4.2.1. Mortality
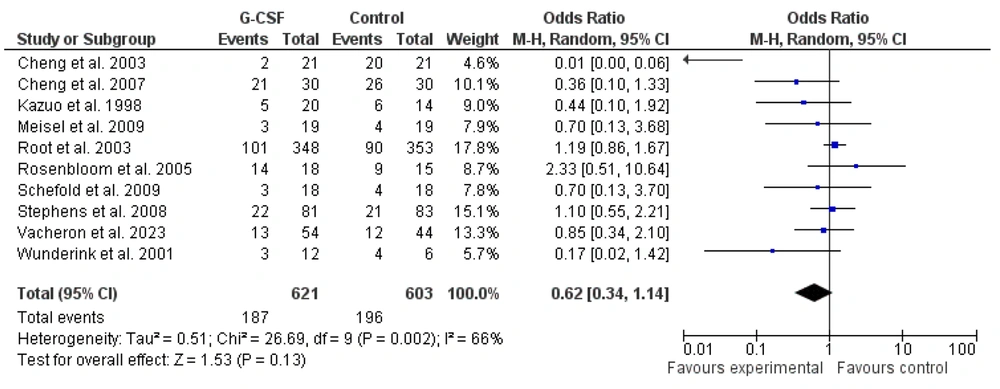
To determine the effects of G-CSF administration on the mortality of sepsis and septic shock patients hospitalized in the ICU, 10 published clinical studies from 1998 to 2023 were selected and included in a meta-analysis. These studies specifically examined the impact of G-CSF on mortality rates. Most of the selected studies reported a reduction in mortality rates with G-CSF administration in patients with sepsis and septic shock. However, the study by Root et al. (2003), which had a large sample size (n = 701), reported no effect of G-CSF. Due to its large sample size, this study had the greatest impact on the results of this meta-analysis (19). The meta-analysis was performed using a random effects model, and the results are shown in Figures 2 and 3. The findings indicated that the administration of G-CSF to sepsis and septic shock patients has no significant effect on reducing mortality rates [0.62 (0.34 - 1.14), Z = 1.53, P = 0.130].
4.2.2. APACHE II Scores
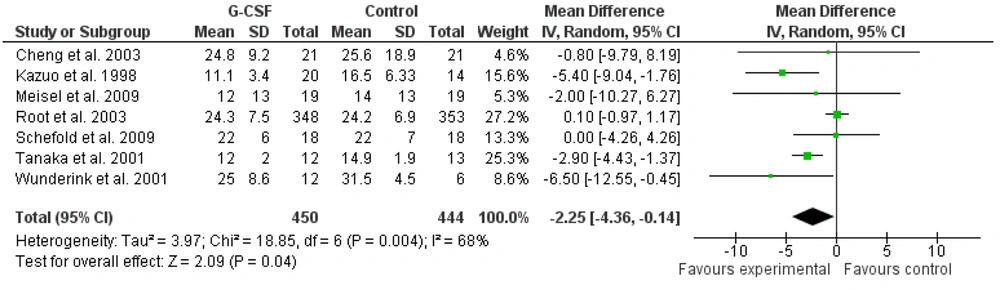
Seven studies that examined the effect of G-CSF administration on APACHE II scores in sepsis and septic shock patients were included in the meta-analysis. Similar to the mortality outcome, the study by Root et al. (2003) had the greatest impact on the results of this meta-analysis due to its large sample size (19). The meta-analysis of the effects of G-CSF administration on APACHE II scores in sepsis and septic shock patients hospitalized in the ICU indicated significant effects (Figures 4 and 5). The administration of G-CSF was associated with a decrease in APACHE II scores [-2.25 (-4.36 to -0.14), Z = 2.09, P = 0.04].
4.2.3. Sequential Organ Failure Assessment Scores
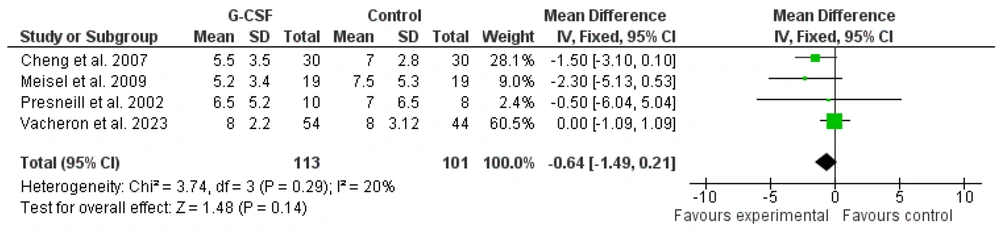
Four studies that reported the SOFA scores of patients were included in the meta-analysis using a fixed effects model. The results indicated an insignificant effect of G-CSF administration on SOFA scores in sepsis and septic shock patients hospitalized in the ICU [-0.64 (-1.09 to 1.09), Z = 1.48, P = 0.14; Figures 6 and 7].
4.2.4. Intensive Care Unit Stay Length
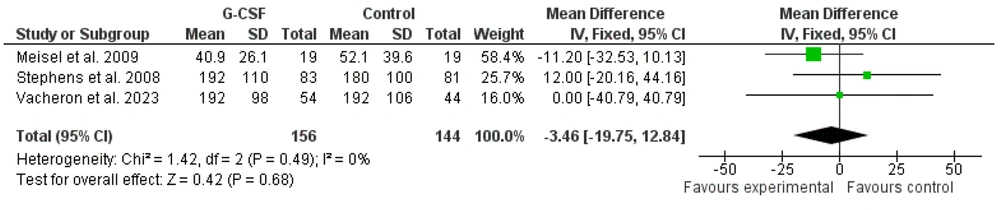
Intensive care unit stay length in sepsis and septic shock patients following G-CSF administration was reported in three studies included in the meta-analysis. The meta-analysis of the effects of G-CSF administration on ICU stay length in sepsis and septic shock patients indicated no significant effect [Figures 8 and 9; -3.46 (-40.79 to 40.79), Z = 0.42, P = 0.68].
5. Discussion
The G-CSF is a glycoprotein that stimulates the production of granulocytes and is produced by immune cells such as macrophages (28). Its analogues, such as filgrastim and lenograstim, are commercially available and effective in enhancing the activity of neutrophil precursors and their maturation (29). Considering that neutrophils play a crucial role in defending the host against bacterial infections (30), researchers have investigated the effects of G-CSF and its pharmaceutical analogs in sepsis conditions. Contradictions in the findings of these studies prompted us to conduct this meta-analysis to investigate the effect of G-CSF in sepsis and septic shock patients. The results of the present meta-analysis indicated no significant effect of G-CSF on mortality, SOFA scores, and ICU length of stay in sepsis and septic shock patients. However, administration of G-CSF was associated with a decrease in APACHE II scores in these patients.
5.1. Granulocyte-Colony Stimulating Factor and Mortality Rate
Sepsis-related deaths are common, with an estimated 11 million sepsis-related deaths (approximately 20% of all deaths) worldwide in 2017. Although there is a decreasing trend in sepsis-related mortality compared to the 1990s, sepsis-induced mortality remains a major concern in the health systems of most countries (31). For this reason, reducing its rate is central to research related to treatment approaches for this disease. In the current study, the effect of G-CSF on mortality was investigated in a meta-analysis, and the results indicated that it had no effect on reducing the mortality of sepsis and septic shock patients hospitalized in the ICU.
However, some studies have reported that G-CSF administration to patients with septic shock and sepsis is associated with reduced mortality. For example, Cheng et al. reported an 85% reduction in the mortality rate of septic shock patients due to melioidosis with G-CSF administration (14). Interestingly, the same authors in 2007 reported no significant effect of G-CSF administration on the mortality rate of severe sepsis patients (15). Similarly, Meisel et al. mentioned the lack of significant effect of GM-CSF on the mortality rate of sepsis patients. However, they found that GM-CSF administration can reduce ICU length of stay and shorten the time of mechanical ventilation (17). The study conducted by Root et al. had the largest weight (17.8%) in the present meta-analysis due to its large sample size, and their findings indicated no significant difference in the mortality rate of severe sepsis patients receiving filgrastim compared to the placebo group (19). Therefore, it seems that adjuvant treatment of sepsis patients with G-CSF has no effect on their mortality rate.
5.2. Granulocyte-Colony Stimulating Factor and Sequential Organ Failure Assessment, APACHE II Scores
One of the interesting findings of this meta-analysis was the significant effect of G-CSF administration in reducing APACHE II scores in sepsis and septic shock patients (P = 0.04). However, G-CSF did not show a significant effect on the SOFA scores of these patients. Considering that APACHE II is a measure used to evaluate the severity of the disease (32), it can be said that adjuvant treatment of sepsis and septic shock patients with G-CSF can reduce the severity of sepsis. On the other hand, SOFA measures dysfunction or failure of organs (33), and the lack of significant effect of G-CSF on SOFA scores indicates its ineffectiveness in reducing vital organ dysfunction.
The reduction in disease severity can be attributed to the mechanism of action of G-CSF in improving the function of immune system cells by increasing the number of neutrophils. As the most abundant white blood cell, neutrophils play an important role in the immune system, and it has been found that initial neutrophil counts are associated with increased severity of sepsis (34, 35). Neutrophils are the first line of innate defense against infections and pathogens (36), and any disruption in their production can lead to serious consequences such as sepsis (37). Sepsis is associated with deregulation of neutrophils, and sepsis and septic shock patients usually show a low neutrophil count. The G-CSF plays a pivotal role in regulating the production and survival of neutrophils, and its administration has been recommended in studies to improve neutrophil counts (38). Interestingly, improvement in neutrophil count in severe sepsis patients after G-CSF administration was reported by Cheng et al. (15). Therefore, according to the role of neutrophils in fighting infections and the improvement of their counts after G-CSF administration, it can be said that the reduction of APACHE II scores (severity of sepsis) can probably be attributed to the improvement in the number of neutrophils by G-CSF. However, more research is needed in this area.
5.3. Conclusions
In general, it is concluded that the administration of G-CSF as an adjunctive treatment for sepsis and septic shock patients does not affect patient mortality rates, dysfunction of vital organs, or the length of stay in the ICU. Nevertheless, it can be effective in reducing the severity of sepsis, as indicated by APACHE II scores.
5.4. Limitations
It is worth mentioning that the selected studies exhibited relatively high heterogeneity (> 60%) for the outcomes of patient mortality and APACHE II scores. A random effects analysis was used to reduce the risk of bias.