1. Background
Chronic inflammation in adipose tissue causes metabolic diseases (1). Numerous studies have shown that adipocyte enlargement is associated with increased expression of inflammatory factors and decreased levels of anti-inflammatory factors (2, 3). Increased secretion of inflammatory factors is the major cause of mild inflammation in the adipose tissue. Some studies have identified immune cell infiltration into adipose tissue, which secretes inflammatory factors, as a key factor in the inflammation (4-6). For unknown reasons, under inflammation conditions, M2 phenotype macrophages, which are mainly anti-inflammatory in lean conditions, are replaced with a variety of M1 phenotype macrophages, which secrete the inflammatory cytokines, and its result is justification of pathogenesis of obesity-induced insulin resistance (7, 8). CD86 is from the M1 phenotype group and CD206 is from the M2 group phenotype. There are pieces of evidence where in the obese condition the CD86 levels increase (9, 10). CD206 plays a role in cell repair and recovery (11-13). Aerobic training (14) are recommended as an appropriate way to prevent and treat inflammation-induced problems. Sympathetic mimicking phytochemicals can also decrease the inflammation process by increasing lipolysis and altering adipose tissue metabolism (15, 16). Octopamine (O) is an endogenous amine that, due to its close association with norepinephrine, can mimic the sympathetic function in many tissues and act as neurotransmitter invertebrates. O affects adrenergic and dopaminergic systems (17). The effects of O are included antioxidant (18), anti-inflammatory (19), weight loss, and fat burning (20).
2. Objectives
In regards to noted sentences the present study aimed to review the effect of AT and O supplementation on macrophage markers in white adipose tissue of rats poisoned with deep-fried oil (DFO).
3. Methods
A total of 30 male Wistar rats, aged 20 weeks, weighing 300 - 350 g were purchased from Razi Institute (Iran). The rats were kept in standard conditions for one week to adapt to the laboratory environment. Rats were divided into groups including healthy control, DFO control, AT + DFO, O + DFO, and AT + O + DFO. During four weeks the O groups received 81 μmol/kg octopamine for five days/week intraperitoneally and AT groups performed training with moderate intensity.
3.1. DFO Preparation
A total of 8 liters of sunflower oil was used to produce heated oil. The oil was heated for four consecutive days for eight hours at a temperature of 190 - 200ºC and chicken nuggets, potatoes, poultry, and protein products (sausages and sausages) were dipped into the oil every 30 minutes, according to references, and finally, the fourth-day oil was kept for use as a poisoning intervention and fed orally to rats for four weeks (21).
3.2. O Administration
O supplements were purchased from the Sigma Aldrich Company. During four weeks the O groups received 81 μmol/kg O supplementation (dissolved in 9 % normal saline) for five days/week intraperitoneally.
3.3. AT Protocol
To adapt to training, the rats ran on the treadmill for 20 minutes at a speed of 9 m/min, for one week. Training duration was 20 minutes and the intensity of training started with 16 m/min in the first week and reached to 26 m/min on the last training session (forth week). For the start of training the rats warmed up for five minutes at a speed of 7 m/min and at the end of the main training, they cooled down for five minutes at a speed of 5 m/min.
3.4. Sacrifice and Tissue Sampling
Forty-eight hours after the last intervention, rats fasted for 8 - 10 hours and were weighed before sacrificing. The anesthesia was inhaled with chloroform. Blood samples were taken from the left ventricle. The white adipose tissue was then rapidly removed, washed with saline, and placed in microtube. Samples were transferred into a nitrogen tank and then stored at -80ºC until cell analysis.
3.5. Cd-86 and Cd-206 Measurements
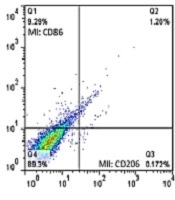
Cell isolation was done from adipose tissue by tripsine 0.25% and DNase in 37ºC for 30 min and centrifuged at 4ºC, 1200 RPM for 5 min. Single cells were used to identify monocyte/macrophage lineage surface markers CD206 and CD86 antibodies. Flow cytometric analysis was performed using a Navios Flow Cytometer and the Kaluza analysis software (Beckman Coulter, Milan, Italy), evaluating a total of 5 × 105 cells and detecting more than 30 events in the smallest subset investigated, according to consensus guidelines on the minimal residual disease.
3.6. Histological Analysis
Samples were placed into a 30% sucrose solution prepared in PBS for three days at 4ºC. Samples were removed from the sucrose solution and placed directly into a “cryomold” containing sufficient optimal cutting temperature compound (OCT) embedding media to fully submerse the tissues. The entire cryomold was then placed onto the cryobar inside the cryostat and, by using a press tool. Frozen tissues were recovered from the cryomold and affixed to a textured cryosectioning plate using OCT as an adhesive. Each section was then placed in separate dry microscope slides (thickness: 8 µm). Placing sections directly into PBS quickly dissolves any residual OCT and ensures that the sections stay hydrated; the hydration step improves overall image quality. H & E staining was done and the pictures were captured and analyzed by Image J to count adipocyte cells.
3.7. Statistical Model
In the descriptive section, the mean (M) and standard deviation (SD) indices were used. Inferential statistics and hypothesis testing were analyzed using two-way analysis of variance for independent groups. Based on this model, at first, the effect of AT and O alone on the outcome was analyzed and the interactive effects of AT and O on outcomes were tested. To determine the effect of poisoning with DFO on outcomes of the study, independent sample t-test was performed for independent groups of healthy control and DFO control groups. Significance level was considered P < 0.05 for all calculations.
4. Results
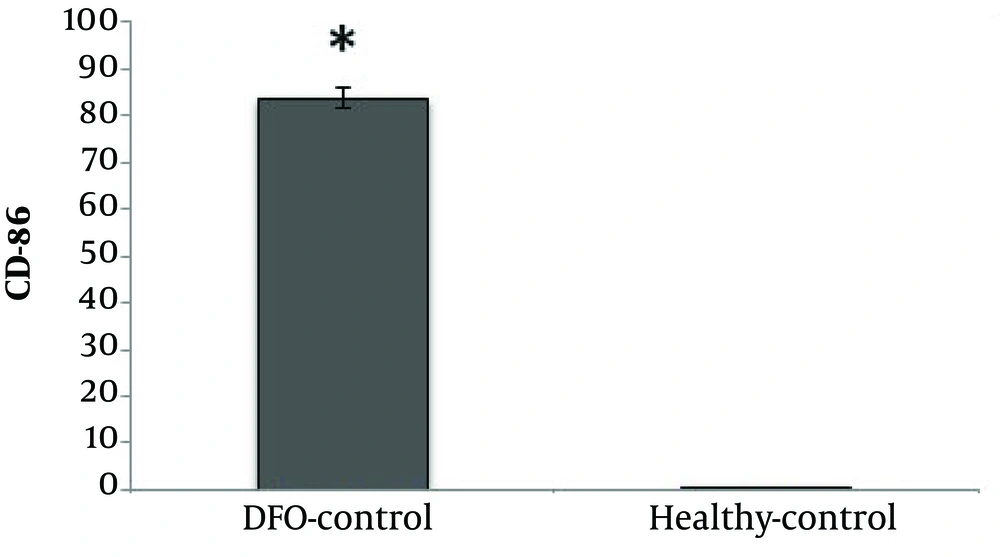
CD86 cell percentage was significantly higher in the DFO group compared with the healthy control group (P = 0.001) (Figure 1).
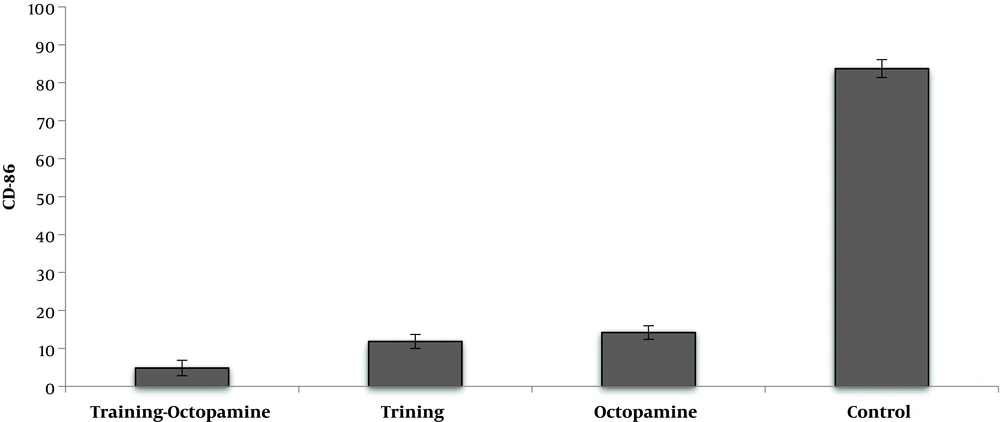
AT significantly reduced CD86 cell percentage compared to untrained rats (F = 2412.72, P = 0.001, µ = 0.992). O supplementation significantly reduced CD86 cell percentage compared to their control group (F = 2142.85, P = 0.001, µ = 0.991). Interaction of AT and O on reduction of CD86 cell percentage was significant, so that AT simultaneously with O consumption had more effect on reduction of CD86 cell percentage rather than each one alone (F = 1432.44, P = 0.001, µ = 0.986) (Figure 2).
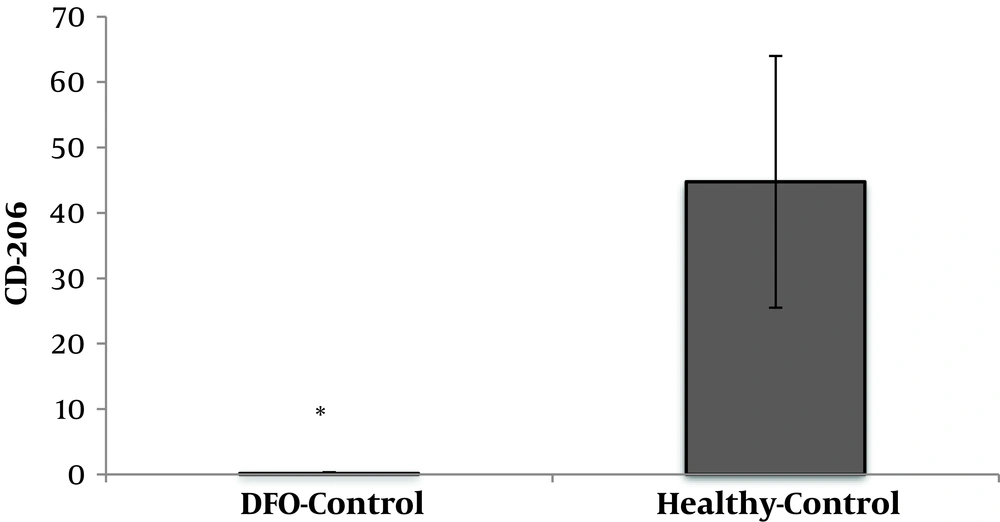
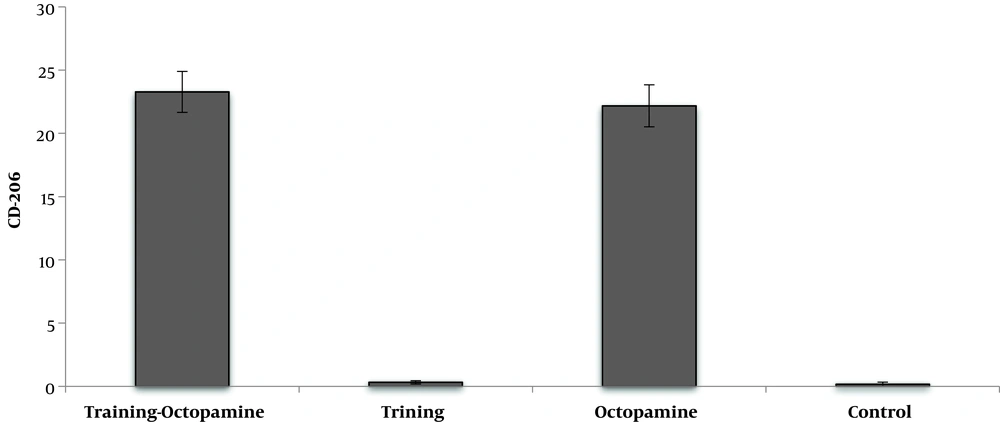
DFO consumption significantly reduced CD206 cell percentage in DFO rats compared to healthy control rats (P = 0.001) (Figure 3).
AT had not significant effect on the CD206 cell percentage (F = 1.72, P = 0.204, µ = 0.079). O supplementation significantly increased CD206 cell percentage compared to no supplement groups (F = 2228.75, P = 0.001, µ = 0.991). The CD206 cell percentage in AT + O group was significantly higher than the training and control. AT + O had no significant changes compared to the O group (F = 0.97, P = 0.335, µ = 0.047) (Figure 4).
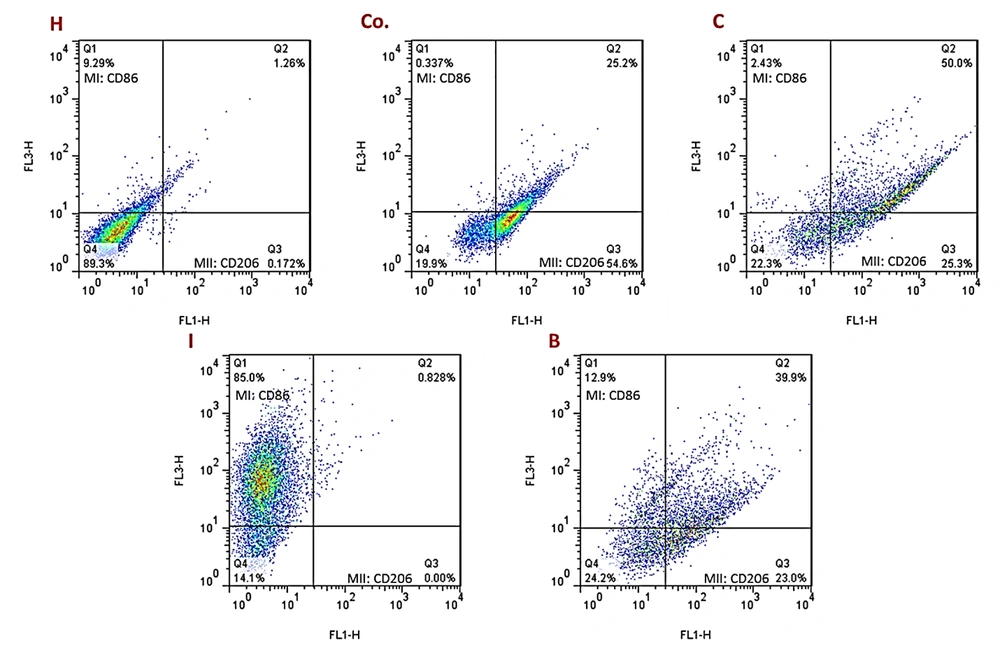
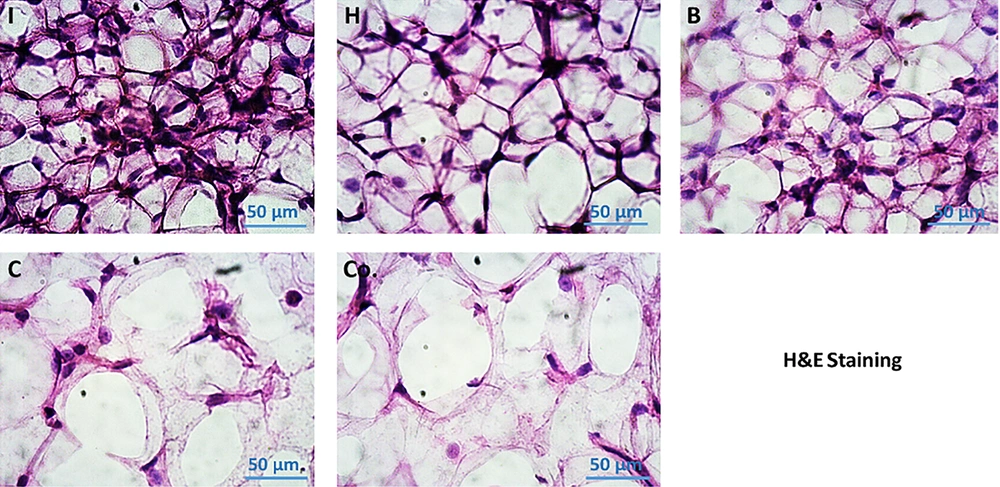
The Flowcytometric assessment of MI and MII macrophage markers in adipose derived cells in different experimental groups of the present study are shown in Figure 5. Results showed that the number of adipose cells in visceral adipose tissue was higher in the DFO control group than the other groups. In addition, the results showed that in a certain unit of tissue, this cell population was also increased in the AT and O groups compared to the healthy control group. However, these two groups had significantly less adipose cells than the DFO control group. In the AT and O groups, these cells were significantly reduced, which was not significantly different from the healthy control group. Based on the histological images, it was found that in the groups that had increased adipose cell population, the diameter of each adipose vacuole decreased, however, in the groups that had decreased cell population, the adipose vacuoles diameter increased. It seems that as the amount of DFO in the groups increases, the adipose cell population first increases and then the cells begin to become obese and hypertrophy, which this trend indicates a gradual increase in adipose tissue (Figure 6).
5. Discussion
The findings of the present study showed that DFO consumption significantly increased CD86 cells and decreased CD206 cells. Numerous studies have suggested the role of adipose tissue macrophages in inflammation (5, 7, 22, 23). The increase in the number of adipose tissue macrophages can be justified by two distinct mechanisms. The first is a change in macrophage recruitment from monocytes and the second a local proliferation of macrophages called from adipose tissue. It has been suggested that the largest population of macrophages in adipose tissue of obese mice is the M1 phenotype macrophages, which is associated with increased expression of inflammatory mediators such as tumor necrosis factor-α (TNF-α) (12). In contrast, the largest population of macrophages in lean conditions is M2 phenotype members, which is associated with the gene expression of anti-inflammatory proteins such as IL-10 (7). In this study, CD86 from M1 phenotype and CD206 from M2 phenotype were studied and the findings obtained from DFO consumption are consistent with the findings in this area. The consumption of DFO significantly increased the CD86 population, while the CD206 population reduced significantly, which indicates a change in macrophage population pattern under obesity conditions. Fatty acid increase in adipose tissue seems to activate the TLR4, NF-κB, IKKβ, and JNK, which each of these substances stimulate inflammatory cytokines such as TNF-α, IL-6, and iNOS (24). These inflammatory cytokines and proteins involved in the feedback loop between adipocytes and circulating monocytes increase polarization of M1 phenotype macrophages and increase their penetration into adipose tissue. Accordingly, an increase in CD86 may be justified by consumption of DFO. Another finding of the present study showed that AT significantly reduced CD86 levels in rats fed DFO, however, AT had no significant effect on CD206 levels. AT is associated with anti-inflammatory responses in various organs such as skeletal muscle, liver, and adipose tissue (25). AT can reduce visceral fat mass and subsequently reduce the production of inflammatory adipokines, decrease in expression of quasi-tail receptors in monocytes and macrophages, as well as produce anti-inflammatory molecules from leukocytes and skeletal muscle (26, 27). Inhibition of monocyte/macrophage infiltration into adipose tissue and alteration of macrophage phenotype into adipose tissue are results of training (27-29). The anti-inflammatory effect of training may reduce inflammation by induction of change of M1 macrophage to M2 macrophage levels in adipose tissue as well as by reducing adipose tissue permeability to macrophages and protecting against chronic inflammatory diseases. Accordingly, it has been shown that running on the treadmill (in obese mice) decreased the expression of M1 macrophage marker and increased M2 macrophage marker (28). On the other hand, decreased TNF-α mRNA expression and CD11c levels after training in HFD mice have been reported (30). According to the above findings, it seems that the decrease of CD86 in this study can be attributed to the decrease of fat cell size in the AT group compared to the DFO control group. As noted, due to the decrease in adipose cell size, the rate of M1 phenotype macrophage entry reduces, which was observed in the present study. Nonetheless, AT had no significant effect on CD206 in adipose tissue. In a study, it has been shown that in mice with HFD, training couldn’t increase the levels CD206 cells, however, it has even decreased (14), which researchers believe that improving the inflammatory profile in HFD mice with training may be due to a decrease in both M1 and M2 phenotype macrophage in adipose tissue.
Another finding of the present study showed that O administration decreased CD86 levels and increased CD206 in adipose tissue. O enhances the process of lipolysis by activating β3-adrenoceptors in adipose tissue (31). Increased lipolysis in adipocytes attenuates the accumulation of fatty acids and this decrease weakens the inflammatory mediators (24). As a result of the decrease in inflammation, macrophage permeability to adipose tissue decreased and a change in macrophage pattern from M1 to M2 phenotype is observed (7, 25). Interaction of AT and O on reduction of CD86 was significant. Both AT and O appear to decrease the permeability of these macrophages through decreasing adipose cell size and inflammatory mediators. Although in the AT + O group the CD206 levels were higher than AT and O groups alone, the observed interaction was not statistically significant. It appears that the effect of AT and to some extent O is more on the M1 phenotype than on the M2 phenotype.
5.1. Conclusions
The results of this study showed that feeding with DFO changed the permeability of adipocytes to macrophages and increased CD86 entry and decreased CD206. Nonetheless, AT can affect this migration process and reduce CD86 penetration into adipose tissue, although it did not affect CD206 penetration. However, O was able to alter the permeability of adipose tissue to macrophages and reduces the negative effects of feeding with DFO. Interaction of AT and O was significant only on CD86. It appears that AT and O were more influenced on the process of M1 phenotype than M2. Nevertheless, the decrease in adipose cell size may be the main cause of the change in macrophage permeability in adipose tissue. The anti-inflammatory effects of AT and O appears to be justified by these changes. However, further studies are needed.