1. Background
Gastric cancer (GC) is the second leading cause of cancer-related mortality (1). Its incidence shows a wide geographical variation. About half of the total gastric cancer load occurs in East Asia, particularly in China and Japan (2). The low-risk areas include Southern Asia, and North and East Africa (3). Although the incidence of GC is gradually decreasing in many parts of the world, it is still the most common malignancy in Iran (4). There are several intermediate and low-risk populations in geographical areas, while the northern and northwestern regions are high-risk areas for gastric cancer (4). The mean incidence rate of stomach cancer is 10.5 - 12 (5). Based on the previous studies, the incidence of stomach cancer is rising in K&B province, including in Yasuj district (6).
Regarding the marked variation of gastric cancer risk in different geographical areas and striking differences in frequency of possible environmental and ethnic risk factors, research on the gastric cancer etiology in each population should be considered as a priority (3).
GC is a multifactorial disease that develops due to continuous cell damage caused by life-long exposure to different predisposing factors, including carcinogens (7-9). Epigenetic alteration involving tumor suppressor genes mutations, DNA repair genes, and loss of heterozygosis (LOH) A may cause cancerous cell mutation (10, 11). Recent studies have revealed that p53 mutations are biologically and clinically distinct. Genetic alterations in the TP53 gene are fundamental events in both early-stage and advanced stomach tumors (12, 13). Approximately, over 50% of human cancers carry a loss of function mutations in the p53 gene (14) in which, 95% of TP53 mutations occur within the genomic region encoding the sequence-specific DNA-binding domain of TP53 protein (exons 4-9) (15-17). A missense mutation results in a single amino acid change, and this type of point mutation in the DNA-binding domain of p53 can encode a protein that is transcriptionally inactive or that displays altered transcriptional activity compared to the wild-type p53 (18). The majority of p53 mutations in human cancer are missense mutations, sitting within the DNA-binding domain with hot spots at codons G245, R273, and R282 (19). Although hotspot mutants of p53 are frequently investigated, few studies are conducted on R282W. Codon 282 encodes arginine amino acid on the P53 binding site in the central domain during gene expression and makes the minor groove contact (20-22). Mutation in the aforementioned site leads to the exclusion of arginine and breaking the conjunction of central domain with DNA major groove and following a decrease in P53 gene expression (R282W designates an arginine mutated to tryptophan at position 282) (18, 23). R282W P53 mutant can alter the behavior and fate of the tumor cell and is thought to promote the progression of many types of cancer (18). According to a study conducted by Zhang and colleagues, R282W mutation can lead to loss of some wild-type P53 tumor-suppressive activity (24).
Although the incidence of GC is gradually decreasing in many regions of Iran, but it is still the most common malignancy in K&B province (6). Based on the official statistics of the Ministry of Health (2015) the number of patients with GC registered in the K&B province has been increasing (25). However, no study is performed at the molecular level to identify the mutation rate of effective genes in GC, such as the R282W P53 mutation in the patients and healthy populations in K&B province.
2. Objectives
Thus, in the current study, samples were subjected to PCR-RFLP to investigate the R282W P53 gene mutation on exon 8 in GC patients in this province of Southwest Iran.
3. Methods
3.1. Study Area
K&B Province (in the Southwest of Iran) is located at an average altitude of 1200 meters high above the sea level in the close proximity of the Dena Mountain. The weather varies from semi-arid and warm in western areas to cold and humid in the north. Its population is estimated to be 750,000, in which 42 and 58% of individuals settle in urban and rural/nomadic areas, respectively.
3.2. Samples Data
This case-control study was conducted on 90 subjects that were divided into two groups (i.e., 45 patients and 45 controls). The samples were randomly collected from the tissue bank of the pathology laboratory of Beheshti hospital in Yasuj city. Demographic data (including age, gender, and etc.) were collected from the medical records of the hospital. The cancerous tissue (based on ICD-10 Coding System: code C16, stages III and IV of the disease) and control samples (no code c16) were transferred to the Cellular and Molecular Research Center and were subjected to DNA extraction.
3.3. DNA Extraction
DNA extraction from Formaldehyde-Fixed, Paraffin-Embedded (FFPE) biopsy samples was carried out using QIAamp DNA FFPE Tissue Kit (Qiagen, cat: 56404) according to manufacturer’s instruction.
3.4. PCR Reaction
The PCR was carried out on the extracted DNA samples. The reaction mixture was provided by adding 12.5 μL Taq 2X Master Mix (Ampliqon), 20 pmol of each primer, 2 μL of DNA template, and deionized molecular grade water up to 25μL. Primers sequences were forward, 5-TGGTAATCTACTGGGACGGA-3 (Tm:58.4); reverse, 5-CTGCTTGCTTACCTCGCTTA-3 (Tm: 58.4) (26). The thermal cycle program consisted of a preincubation step of 5min at 95°C for complete denaturation of DNA followed by 35 cycles at 94°C for 30s, 55°C for 30s and 72°C for 30 s and final elongation at 72°C for 5 min. The DNA product of exon8 was 149 bp.
3.5. RFLP Reaction
After PCR, 16μL of the PCR product was subjected to enzymatic digestion with 2μL of the endonuclease MspI (Fermentas) and 2μL of enzyme-specific buffer overnight at the recommended temperature (37°C). MspI was used to recognize the sequence CC/GG and cut it at the same site even if the internal cytosine was methylated.
MspI cleaves CC/GG at codon 282, generating 87bp and 62bp from the 149 bp the purified DNA product. Mutation at codon 282 resulted in an uncleaved (c.844C>T (CGG→TGG); Arg282Trp), 149 bp fragment, and this feature had been distinguished from that of normal samples on 10% polyacrylamide gel. The presence of the uncleaved 149 bp fragment indicated that there are mutations in the corresponding samples.
3.6. Statistical Analysis
Data were analyzed by descriptive statistics, including frequency and percentage. Analytical evaluations were performed using the chi-square test. All data were analyzed using the SPSS software version 17. Statistically significant was defined at P-value < 0.05.
4. Results
The demographic and clinical characteristics of the patient and normal groups are described in Table 1. There were 27 (60.0%) males and 22 (48.9%) females with a mean age of 52.07 ± 15.4 years for GC patients. For the control group, 18 (40.0%) were males and 23 (51.1%) females, with a mean age of 47.13 ± 15.12 years. The results showed that 26/45 (57.8%) of the patient group and 24/45 (53.3%) of the control group were illiterate. According to the results, there was no significant difference concerning the education levels, age, and gender between the two groups (Table 1).
| Variables | Case, No. (%) | Control, No. (%) | P-Value |
|---|---|---|---|
| Gender | 0.397 | ||
| Male | 27 (60.0) | 22 (48.9) | |
| Female | 18 (40.0) | 23 (51.1) | |
| Age, y | 0.399 | ||
| < 40 | 12 (26.7) | 18 (40.0) | |
| 40 - 60 | 19 (42.2) | 18 (40.0) | |
| 61 - 80 | 13 (28.9) | 9 (20.0) | |
| > 80 | 1 (2.2) | 0 (0.0) | |
| Education | 0.906 | ||
| Illiterate | 26 (57.8) | 24 (53.3) | |
| Literally | 19 (42.3) | 21 (46.7) |
The frequency of R282W P53 mutation in the control and tumor samples is shown in Table 2. The prevalence of R282W P53 on exon 8 in the cancer group was 17.8% (8/45), while no mutation was found in the control group (0/45) (P = 0.006).
| Group | Mutation, No. (%) | No Mutation, No. (%) | P-Value |
|---|---|---|---|
| Case | 8 (17.8) | 37 (82.2) | 0.006 |
| Control | 0 (0.0) | 45 (100.0) | |
| Total | 8 (8.9) | 82 (91.1) |
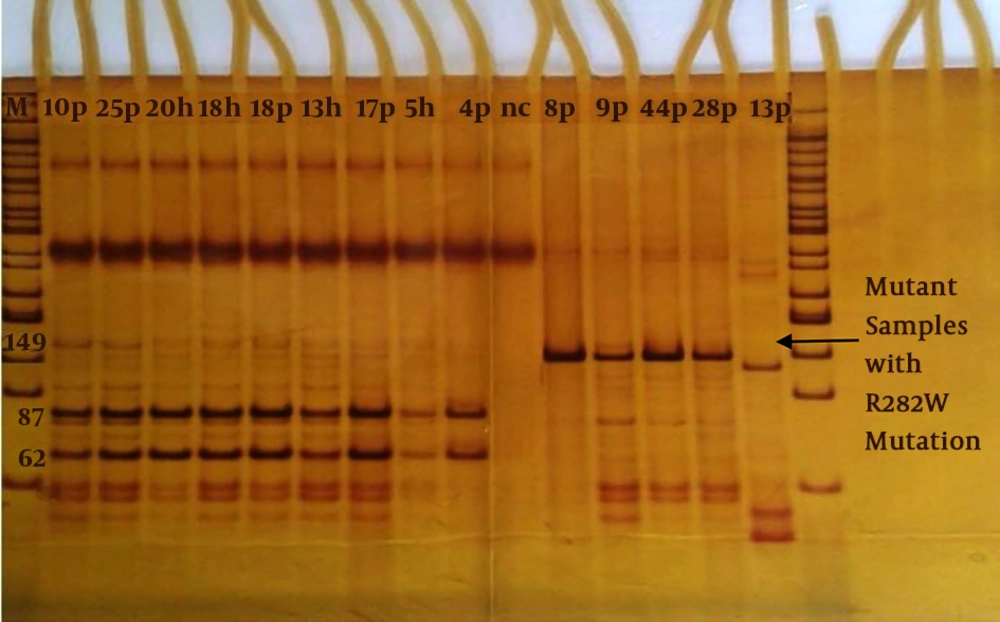
Detection of the mutation of R282W P53 by the MspI restriction enzyme is shown in Figure 1. Besides, we arranged positive (with 282 mutations) and negative (wild-type) controls in Figure 1.
Detection of R282WP53 gene mutation by MspI restriction enzyme on 10% polyacrylamide gel. M; Marker 50 bp, Patient; P, h; health, nc; negative control. Normal samples (87 and 62 bp): 10P, 25P, 18P, 17P, 4P and 20h, 18h, 13h, 5h. Mutant samples with R282W mutation (149 bp): 8P, 9P, 28P 44P and 33p.
5. Discussion
P53 gene mutations are one of the most frequent alterations in human cancers (27). Mutation at codon 282 of the P53 gene is identified as a hotspot mutation for GC (28). The R282W mutant is associated with earlier onset of the familial cancers and poorer outcomes of cancer patients (24, 29).
According to the results, there was a significant association between GC and R282W mutation in the studied population in the Southwest of Iran. Abdullah and colleagues showed that 20% of gastric patients in Kashmir Valley harbor R282W mutation of P53 gene (30), which is in accordance with the present study. Fischer and colleagues found a strong association between R282W P53 mutation and GC in Toronto, where P53 mutation was identified in 40% of cases (31). According to the AACR (American association cancer research) project, P53 was altered in 42.67% of GC patients with P53 Codon 282 Missense present in 1.78% of all GC patients (32). Also, the results of Juvan and colleagues found R282W mutation in 23 nucleotide changes at the P53 gene on exon8 in Slovenian patients with GC (33). This study in accordance with the previous studies, which reported a significant association between the loss of heterozygosity (LOH) at R282W loci and GC. It is well known that certain types of mutations (LOH) do not produce stable proteins, and protein overexpression may be due to the results of the stress environment in GC (28).
Exon8 is an evolutionarily conserved region of P53, and the previous studies showed that 80% - 90% of all P53 mutations in a variety of human malignancies occur here (34). The higher frequency of R282W P53 mutation reported in GC is also reported for some other malignancies. Rashid and colleagues found a significantly higher frequency of R282W P53 mutation (58%) between Chronic myeloid leukemia patients in the Indian population (23). Javid and colleagues found that 62% of patients with Non-small cell lung cancer were positive for R282W P53 mutation in India (35). Besides, point mutations at codon 282 are mutational hotspots reported in hematologic diseases, including Burkitt’s lymphoma (36), Myelodysplastic syndrome (37), T-cell leukemia (38), Lymphoid leukemia (39) and etc.
Analyzing mutation of the P53 in our neighbors and other regions of Iran and other prevalent cancers could be done to compile an inclusive databank on the mutation of P53 and its association with poor prognoses of the disease (40). Karim and colleagues found a significant correlation at exon8 (21.04%) with the P53 alterations in adenocarcinoma of GC (41). The Saffari-Chaleshtori and colleagues study found no mutation in exon8 of the P53 gene in gastric patients Shahrekord city (42). Abbasi and colleagues reported a mutation rate of 6.7% in p53 in the bladder of patients with cancer in Kermanshah city (western are of Iran) (43). Lohrasbi Nejad and colleagues found 4 mutations at the P53 gene (codons: 140, 142, 184 and 248) in colorectal cancer patients in Kerman province (40). However, these studies mentioned to many differences in the incidence of gastrointestinal cancers in various cities of the country (44), but did not provide clear information about R282W P53 mutations. The current study is the first study that specifically examined the R282W P53 gene mutation on exon 8 in GC patients, in K&B Province. The differences in the prevalence of P53R282W gene mutation in various reports may reflect the multitude of factors, including environmental factors, geographic region, race and ethnicity, detection methods, sample size, stage of cancer, etc. (27, 40, 45, 46).
Many studies investigated P53 gene mutations in neighboring provinces, however, no systematic study is performed to distinguish mutants with R282W alterations in GC. The present data are the first report on the R282W P53 abnormalities in GC patients from K&B province. We suppose that a high prevalence of p53 mutation might be related to genomic profiles, environmental factors, or lifestyle-related factors such as H-pylori infection, diet, sunlight, etc. in K&B province, Southwest Iran with nomadic populations and mountainous weather (47-49). Recently, some studies reported that high altitude and increased exposure to ultraviolet (UV) radiations of sunlight, (level of ultraviolet radiation increase by about 10% with every 300 m), may be related to the higher incidence rate of cancers (50, 51). UV radiation has been shown to induce the expression of DNA damage and is known to produce signature mutations in the p53 gene in human (52, 53). Also, there are more than 138 medicinal plants, as the most important sources of herbal food that have been used ethnomedicaly by local people in K&B province for medical and food purposes (54, 55), but anti-cancer or cancerous effects of these plants in humans have not been well studied yet (56). For example, Dorema aucheri (Bilhar) grows in the Southwest of Iran and is routinely consumed by the people in K&B province (57, 58). The biochemical analysis showed that D. aucheri compounds might have carcinogenic and genotoxicity effects on the human cell lines (57, 59).
This study encountered some limitations. Sampling was restricted to those patients on stages III and IV, so it was not possible to increase the sample size. Furthermore, samples were FFPE, and a number of them did not give an amplifiable DNA. Some samples did not show any mutation, which may be because they had not passed stage IIIA or they may have had a mutation in other exons, except exons 5-8. Although, passing this stage did not guarantee the mutation of the P53 gene.
In conclusion, the results of this study indicated that the R282W P53 gene mutation in exon 8 may play an important role in the development of GC in K&B province (Southwest Iran). It is advised to find more probable mutations through investigating other exons of P53 sequences and by using more sensitive methods such as the sequencing approach. Also, we suggest using fixatives other than formalin to preserve the quality of DNA in pathology laboratories for future analysis of molecular investigations. The P53 mutation is of crucial importance in the success of the treatment plan of cancer patients. The current chemotherapy or radiotherapy methods for cancer are completely dependent on the P53 function because they induce the intrinsic pathway of apoptosis only when P53 is normal (60).