1. Background
The self-healing ability of a skeletal muscle does not suffice and fails to repair the damages such as trauma, congenital disabilities, myopathy, and tumor (1). The disconnection of nerves and muscles for a long time would result in removing the positive effects of nerve impulses and neurotrophic factors on protein synthesis in skeletal muscle, thereby destroying a large muscle mass (2). Furthermore, ischemic damages cause blood supply loss in muscle tissues and thus lead to cell death and muscle mass loss (3). Aging also reduces muscle mass and strength. In this regard, several factors (namely decreased protein synthesis, increased apoptosis, and decreased replacement of dead cells by living cells) are involved in the destruction of skeletal muscles by aging (4, 5). Nowadays, the alternative construction of each muscle has been considered in tissue engineering. Successful approaches have been put forth to introduce the types of myocardial stem cells affected by ischemia and necrosis and convert them into myocardial muscle cells (6). However, it is hoped that patients would benefit from the alternative heart to restore the pump activity of the attacked heart in the near future. In recent studies, muscle repairing has been considered as a repetition of muscle development.
The construction of skeletal muscle begins with the adhesion of myoblasts to form the myotubes on embryonic day 9. Then myotubes adhere to each other to form myofibrils on the embryonic days 11 and 12. Some myoblasts do not adhere and continue their proliferation and differentiation up to the embryonic days 15 - 17; hence, they make smaller secondary fibers. Meanwhile, the basement membrane is to surround each fiber. Then lunar cells are considered as mononuclear cells placed between the basement membrane and the fiber plasma membrane. Lunar cells are turned off at the end of their growth after birth; however, they will be activated once more if the muscle tissue is damaged. In this case, they resume their mitotic activity, repair damaged fibers, or make new fibers (7).
During the 1990s, tissue engineering was introduced and developed rapidly. Tissue engineering can be used to repair, preserve, or improve tissues and organs (8). This type of engineering is underpinned by three main biological factors, including extracellular matrix, cells, and biomolecules. One of the main essential characteristics of tissue engineering scaffolds is having a porous network to feed the cells, formation of extracellular matrix, and angiogenesis (9, 10). Acellular biological scaffolds are mainly used to reconstruct damaged tissues in regenerative medicine (11). The Urinary system and bladder are susceptible to various pathological conditions from embryo development to adulthood. Several synthetic materials and natural tissues are examined to detect the renovation of bladder defects. A source of cells having the potentials to build muscle fiber is required for cell therapy and tissue engineering of destructive muscle diseases. In this regard, one of the appropriate cell lines for cell therapy is bone marrow mesenchymal stem cells (BM‐MSC). Recent studies have documented that 5-Azacytidine, which essentially acts as an anti-tumor agent and is also used to treat cancer, can induce the mesenchymal stem cells to skeletal myogenic cells (12, 13).
2. Objectives
The present study aimed to evaluate the effect of these factors on the expression of skeletal muscle-specific genes on typical bladder scaffolds.
3. Methods
3.1. Animals
Male Wistar rats were prepared from the Ardebil University of Medical Sciences and then transported to the animal house. The rats were kept in a 12-hour light/12-hour dark cycle at a mean temperature of 21°C and were fed daily with a special diet. In each phase of this study, the approved principles on working with laboratory animals were observed in accordance with the guidelines issued by the Ethics Committee of the Mohaghegh Ardabili University of Ardabil (Iran) (code: IRUMA 39365).
3.2. BM‐MSCs Preparation, Characterization, and Culture
Femoral and tibial bones were dissected from rats, and the muscles and soft tissues were removed. They were then transferred to the falcon tubes containing Dulbecco’s modified eagle medium (DMEM). The tubes were placed on ice and transferred under a sterile hood. Next, the two ends of the bones were cut, and the bone marrow was removed using a syringe and a needle (14) and transferred to new falcon tubes. To remove the remaining red blood cells, falcon tubes containing bone marrow were centrifuged at 1500 rpm for 4min. The resulting cells were immersed in 1 mL of a new medium.
The cell suspension was cultured in the DMEM medium with low glucose percentage, 10% fetal bovine serum, 100 units per mL of penicillin, and 100 µg/mL of streptomycin in a cell culture flask incubated at 37°C. The supernatant of the cells containing floating blood cells and nonsticking cells was discarded after 24 hours, the new medium was then added, and cultivation was continued under conditions similar to cell proliferation. The cellular medium was renewed every other three days. After eight days, the cells covered 80% of the flask bottom. Then, for cell proliferation, the first cell passage was performed using EDTA/trypsin, and this process continued until passage 3. Passage 3 cells were used to study the differentiation process (15, 16).
After three weeks of culture, BM‐MSCs were subjected to Oil Red O staining for adipogenesis and Alizarin Red for the matrix mineralization of osteogenesis. To confirm the phenotype of BM‐MSCs, the cells at passage 3 were subjected to flow cytometry to determine the surface expression of CD29, CD90, CD105, CD34, and CD45 markers (BD Biosciences, USA), as described by the manufacturer. Moreover, the cells stained with FITC-conjugated IgG1κ, IgA, and IgM were used as isotype controls. Flow cytometry was performed using a BD FACSCalibur flow cytometer (BD Biosciences), and the analysis was run with FlowJo V 10 (TreeStar) software.
3.3. Scaffold Preparation
To prepare a sheep bladder decellularized scaffold, the sheep bladder was prepared from the slaughter, placed in 0.9% normal saline, and transferred to the laboratory. The bladder was opened using sharp scissors, divided into equal pieces, and transferred into a falcon tube. Then the physical decellularization of the bladder began at low temperature (17). Next, the physical (18, 19) and chemical (20, 21) combination methods were used for decellularization. To this end, the pieces of the bladder were put in a freezer at -4°C for 24 hours and immersed in distilled water containing 0.1% sterile sodium azide per 6 hours for 10 minutes. After 24 hours, they were put in a freezer for 2 hours and 1 hour at -20°C and -40°C, respectively to be prepared for being placed in liquid nitrogen. The bladders were placed in liquid nitrogen in five 2-minute steps by cryotubes and were then washed with PBS containing 0.1% sodium azide. In this regard, the physically decellularized method was completed. In the chemically decellularized method, all the pieces of the bladder were exposed to 1% sodium dodecyl sulfate (SDS) solution during slow rotation for 24 hours. Then the samples were washed with distilled water and sterilized with 75% ethanol and 0.2% acetic acid, and finally placed in PBS for 24 hours. Then the scaffolds were prepared and stored at -20°C.
3.4. BM‐MSCs Culture On Sheep Bladder Scaffold
The scaffolds were cut into dimensions of 1 × 1 cm2 and were soaked in 70% ethanol for 24 hours. After drying the scaffolds at room temperature, both sides were sterilized by being exposed to UV radiation for one hour. Then the scaffolds were transferred to a 24-well plate, washed with PBS, and treated by PBS in an incubator at 37°C and 5% CO2 for 3 h. First, the 20µl cells at a density of 2 × 105 BM‐MSCs were seeded on the scaffolds. Next, the plates containing scaffolds were incubated for 3 hours to have the complete penetration of cells into the scaffold. Then, 500 µL of the medium and 20% FBS and 1% antibiotics were added to the scaffolds (16).
3.5. Evaluating Biocompatibility of Scaffolds
As mentioned before, BM‐MSCs were cultured on scaffolds to investigate the biocompatibility of the bladder scaffold. The scaffolds cultured with cells at three intervals (days 3, 5, and 7) were transferred to a new 24-well plate and washed with PBS. The MTT solution (3-[4,5-dimethylthiazol-2yl]-2,5-diphenyltetrazolium bromide) at a concentration of 20 mg/mL was added to the DMEM medium. Then the plates were incubated at 37°C with the humidity of 98% and 2% CO2 for 4 h. Afterward, the medium on the cells was slowly removed, and 100 µL DMSO solvent was added to each well to dissolve purple formazan crystals formed by decreased MTT. The optical density (OD) of the obtained color in each sample, which is directly associated with the number of metabolically active cells, was measured by Elisa reader (URIT-660, Germany) at a wavelength of 570 nm. The experiment was repeated three times (22).
3.6. Differentiation of BM‐MSCs Into Myocytes on Scaffold
The process of differentiation of mesenchymal stem cells into myocytes was studied in vitro using the third passage. First, the cells were cultured on the scaffold, the next day, the medium in the wells containing the scaffold was removed, and myogenic differentiation medium was added after rinsing the cell scaffold with PBS. The differentiation medium of myocytes was 5-azacytidine, the treatment lasted for 28 days, and the culture medium was renewed every other two days.
3.7. Assessing the Differentiation of Stem Cells Into Myocytes by Masson’s Trichrome Staining
Masson’s trichrome staining was used to assess the myogenic differentiation of cells on the scaffold. Staining was observed during the second, third, and fourth weeks. To this end, the scaffold containing differentiated cells was embedded in paraffin after dehydration and drying by a freeze dryer and then was sectioned, stained, and assessed by an optical microscope.
3.8. Statistical analysis
All experimental data were analyzed using SPSS software version 16. One-way analysis of variance (ANOVA) was used to analyze the collected data. Moreover, the Tukey posthoc test was used to compare the groups in pair, and P < 0.05 was set as the significance level in all tests, respectively.
4. Results
4.1. Morphological Study of Scaffolds by Electron Microscope
Electron micrographs prepared from the surface of scaffolds by electron microscopy showed the scaffolds with porous structure and interrelated pores (Figure 1).
4.2. Cell Characterization and Culture on Scaffolds
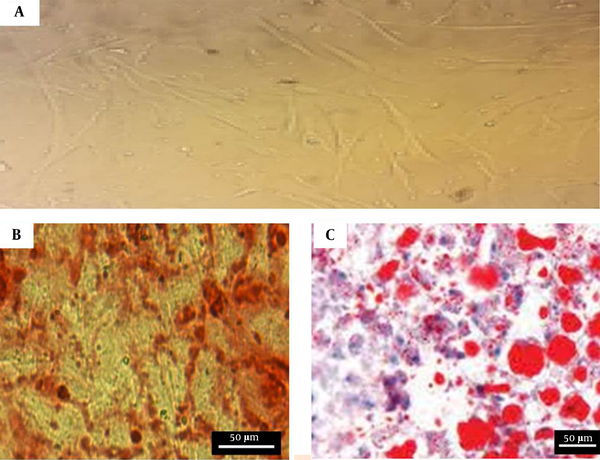
The BM‐MSCs cultures at passage 3 showed a fibroblast-like morphology under an inverted phase microscope (Figure 2A). The cultured BM‐MSCs at passage 3 were performed by the FACS analysis of surface marker expression to confirm the BM‐MSCs’ characterization. The results indicated that BM‐MSCs expressed high levels of CD29 (87.32 ± 2.1%), CD90 (88.53 ± 0.56%), and CD105 (95.41 ± 0.78%); however, the expressions were negative for hematopoietic lineage markers CD34 (2.98 ± 0.12%) and CD45 (2.85 ± 0.12%). Moreover, using Alizarin red S staining and oil red O staining, the cells cultured in the osteogenic and adipogenic medium indicated the mineralization of calcium in osteogenic medium and the accumulation of intracellular lipid vacuole (Figure 2B and C). The morphology and cell junction on the surface of the scaffolds were investigated using electron microscopy images. The images demonstrate successful adhesion between the cells and the scaffolds (Figure 3).
Characteristics of BM‐MSCs: A, BM‐MSCs at passage 3. The cells have a fibroblast-like morphology; B, Osteogenic differentiation potential of BM‐MSCs. Calcium crystals are presented in alizarin red staining; C, Adipogenic differentiation potential of BM‐MSCs. Lipid droplets are shown in oil-red-O staining.
4.3. Biocompatibility of Scaffolds
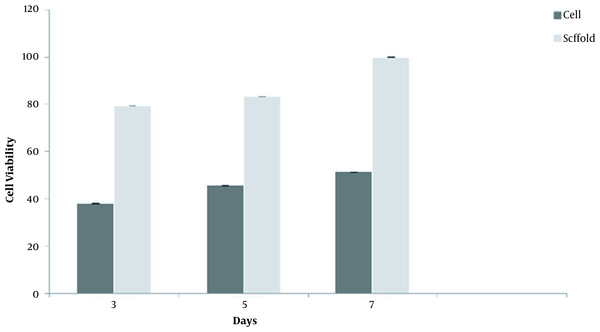
The biocompatibility rate of the cells was determined using an MTT assay based on the reduced activity of the mitochondrial enzyme in BM‐MSCs cultured on the scaffold. As shown in Figure 4, the biocompatibility of the cells on the scaffold was assessed on days 3, 5, and 7. By the end of day 7, the scaffold showed the highest biocompatibility, representing the non-toxicity and suitableness of the scaffold for cell growth (Figure 4).
4.4. Confirming Myogenic Differentiation by Masson’s Trichrome Staining
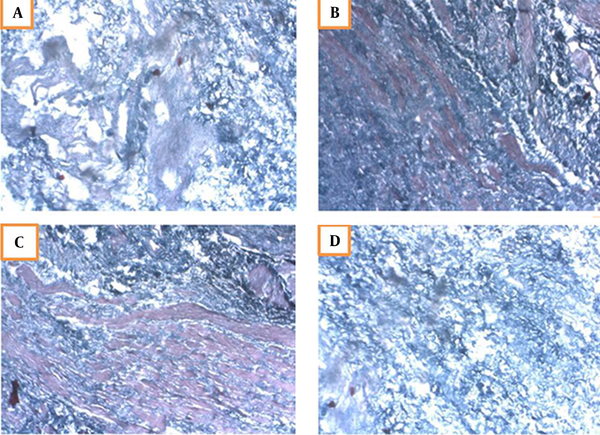
Masson’s trichrome is a specific stain for muscles. The bladder cells cultured on the scaffold were treated with 5-azacytidine for four weeks and then evaluated by specific Masson’s trichrome staining. In this regard, red staining represented the muscle cells. The first signs of differentiation appeared in the second week, and the muscular cells were differentiated and distinguishable at the end of the fourth week (Figure 5). The control scaffold (without cells) was also stained, in which only purple represented the lack of muscle cells.
Masson’s trichrome staining of differentiated muscle cells on the scaffold; A, Second week of differentiation characterizing the first symptoms; B, Red muscle cells are detectable in the third week; C, The cells are completely drawn and differentiated in the fourth week; D, Control scaffolds without cells.
4.5. Expression of Differentiation Genes
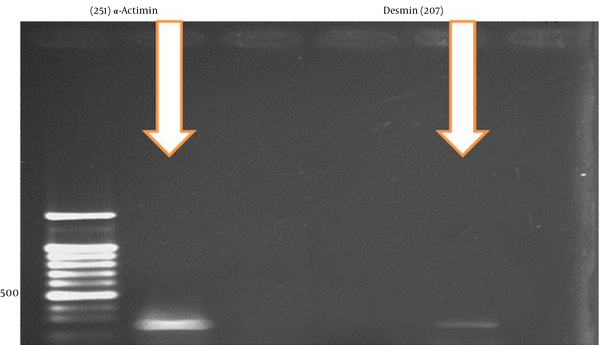
An investigation of the differentiation genes in the fourth week demonstrated that α-Actinin and Desmin genes had the highest expression after 28 days of cell treatment with 5-azacytidine (Figure 6).
5. Discussion
Acellular scaffolds provide an acceptable platform for studying cell behaviors by maintaining the original compositions of the extracellular matrix (ECM). Preparing such scaffolds seems to be the hotbed of discussion in future biological studies due to its broad applications in regenerative medicine and tissue engineering (23). A source of cells with the potentials to build muscle fiber is required for cell therapy and the treatment of degenerative muscle diseases in tissue engineering (21). In recent years, scientists have succeeded in isolating and culturing stem cells mainly from the bone marrow, adipose tissue, and other sources, which can be converted to various types of cells and tissues (24-26). Myogenic potential factors have been investigated in many different studies, according to which various factors such as 5-azacytidine, horse serum, dexamethasone, hydrocortisone, amphotripcin B, and a combination of insulin, transferrin, and selenium are involved in differentiating stem cells into skeletal muscle cells (27). In this regard, 5-azacytidine is a principal demethylating agent, which selectively activates gene expression and affects gene differentiation. Previous studies have proved that it results in the differentiation of mesenchymal stem cells into skeletal myoblasts. Accordingly, 5-azacytidine was used to optimize the differentiation medium condition for skeletal muscles in this study (28). Many studies suggest that cells differentiate into skeletal-like myocytes when incubated in the presence of 5-azacytidine. However, the exact concentration of myogenic differentiation is still unknown, and further studies are recommended in this regard. Jones and Taylor, for the first time, reported that 5-azacytidine induces myogenesis in adult cells and embryonic stem cells (14). Xu et al. (29) examined the effect of 5-azacytidine at a concentration of 10 µM on the differentiation of heart and skeletal muscles from BMMSCs. He observed that alpha-actin and myosin genes increased in skeletal muscles by the end of the second week (29). The skeletal muscle accounted for 40% of adult human body volume. Skeletal muscle diseases encompass muscle weakness, paralysis, and even death (30). Some processes have been performed on the swine urinary bladder matrix as a known biomaterial scaffold consisting of the ECM components, including collagen, laminin, fibronectin, glycosaminoglycans, growth factors, chemokine, and cytokine. Biomaterials in the scaffold should support the adhesion, growth, and proliferation of cells under both in vivo and in vitro conditions (31). Researchers have used mechanical and electrical stimulation to promote myoblast fusion, alignment, hypertrophy, and contractile TEMGs in vitro (32, 33). On the other hand, some structural molecules have been found in the urinary bladder matrix, which has significant moderating effects on cell behaviors after destruction. For example, The RDG short peptide in fibronectin is involved in binding integrin-mediated receptors of cells; however, the acellular scaffolds include various components such as growth factors and cytokines, which facilitate cell growth and cell adhesion to the scaffold. In this regard, of 129 detected proteins, 39%, 11%, and 14% are derived from cytoplasm, secretory proteins, and tissue ECM, respectively. A large group of these proteins plays a vital role in the cell structure (19%) and adhesion (13%), while a smaller group is involved in the stimulation and regeneration of cells (1%).
In this study, fibroblast-like and spindle-shaped cells were removed in the early hours by changing the medium constantly. The cultured Balb/c sticky cells derived from the murine bone marrow include fibroblasts, blood progenitor cells, macrophages, endothelial cells, and fat. Previous studies have indicated that these cells remain in the cell culture and infect fibroblastic cells. The present study indicated that the frequent replacement of the culture medium could prevent the attachment of blood cells and other non-mesenchymal to plastic culture plates. In this method, the pure set of fibroblast-like cells with high proliferative capacity and ability to differentiate to mesenchymal lineages (Adipocytes, chondrocytes, and osteocytes) was obtained within two weeks after the initial culture. In general, the spindle-shaped morphology and clonogenicity of the cells, high proliferative potential, and differentiation into mesenchymal lineages (bone and fat) suggested that the cells obtained in this study are a pure population of mesenchymal stem cells. A hematoxylin-eosin stain was used for the staining scaffold to investigate the presence of the cells on the bladder scaffold, indicating the viability of the cells on the bladder scaffold. Masson’s trichrome staining, a specific stain for muscle, was used to evaluate differentiation in cells treated with 5-azacytidine. The staining results demonstrated BMSCs differentiated into myocytes.
Moreover, the expression of differentiation genes (namely Myod, Actinin, Desmin) treated with a 5-azacytidine inducer after 28 days illustrated the Desmin gene expression on the third week. Desmin is a protein involved in the junction of sarcomeres close to the Z line, and MyoD is a protein playing a vital role in myogenic differentiation. This issue indicates that, after 9 - 10 days of exposure to 5-azacytidine, the cells became multinucleated and oblong, and the MyoD expression increased in the treated cells. Furthermore, Masson’s trichrome staining was used to assess the differentiation of mesenchymal stem cells into myocytes in the fourth week, in which blue, purple, and red represent the collagen fibers, nuclei, and muscle and epithelial cells, respectively. The present study suggest that the treatment of BMMSCs with the 5-azacytidine inducer leads to differentiation into myocytes during 28 days.
5.1. Conclusions
This study demonstrates that BMMSCs on the sheep bladder scaffold treated with 5-azacytidine express muscle genes and differentiate muscle cells.
5.2. Limitation
Lack of facilities to assess differentiation by Western blot analysis.
Suggestion
1) Differentiation assessment by western blots and immunocytochemistry;
2) Confirming differentiation and evaluating myogenic growth factors in-vivo.