1. Background
Spermatogonial stem cells (SSCs) remain in spermatogenesis during their lives. The SSCs are characterized by undergoing both differentiation and self-renewal of cell divisions, resulting in the production of haploid sperm (1). The mechanism underlying SSC proliferation is not yet known. The culture environment is a static system in vitro, and the cells cultured require numerous nutrients and show a higher concentration of reactive oxygen species (ROS) (2). The ROS is involved in apoptosis signaling processes in organelles of the cells, such as mitochondria, under the regulation of Bcl-2 family proteins (3). Lipid peroxidation is caused by free radicals and results in DNA breaks and oxidization of proteins and important molecules. Therefore, the need for antioxidants increases with exposure to ROS (4).
The grape has a significant number of nutritional antioxidants, such as polyphenols and anthocyanins. The grape is rich in oligomeric proanthocyanidins, as the most beneficial groups of plant flavonoids, which have several health-promoting effects, including increasing intracellular vitamin C concentrations, decreasing capillary fragility and permeability, and scavenging free radicals and oxidants (5). Grape seed proanthocyanidins scavenge hydroxyl radical (OH) dose-dependently (6). Importantly, grape seed extract (GSE) has more anti-oxidative effects than vitamins C and E and β-carotene, regulating cell signaling pathways related to apoptosis (7). Additionally, 55% of the procyanidins in grape seeds have over five monomer units (8). Cinnamic acids and benzoic acids as phenolic acids (9) and flavonoids, including anthocyanins, ellagic acid, quercetin, kaempferol, myricetin, proanthocyanidins, and catechins, are considered the most important components of grape that have antioxidant properties (10).
Ascorbic acid, or vitamin C, as a water-soluble vitamin, has been associated with fertility for many years (11). It is an essential molecule that plays a vital role in many biological processes, including immune response and antioxidant activity. Vitamin C plays significant roles in amino acid and collagen synthesis, cholesterol metabolism, and DNA protection by acting as a free radical scavenger (12). It is also involved in many biological activities, including enzymatic reactions catalyzed by dioxygenases that use Fe2+ and 2-oxoglutarate as co-substrates (13). Improving the culture media with sufficient vitamin C amounts leads to a more effective generation of pluripotent stem cells, somatic cells, SSCs (14), and somatic cell nuclear transfer animal embryos (3). Following the addition of vitamin C to the culture medium at certain concentrations, it can act as a growth promoter and increase DNA synthesis and cell proliferation. Nonetheless, significantly high vitamin C levels are cytotoxic and inhibit proliferation and apoptosis (15). Specifically, vitamin C can regulate stem cell identity/behavior, affecting differentiation pluripotency and self-renewal (14).
2. Objectives
This study used the antioxidant properties of grape seed extract (GSE) in comparison to vitamin C to increase the efficiency and function of ovine SSCs. For this purpose, the effects of different concentrations of GSE and vitamin C on the viability and colony formation of SSCs and the expression of apoptotic genes were studied.
3. Methods
3.1. Chemical Agents
The chemicals and culture media were supplied from Sigma-Aldrich (USA), and the plasticware was obtained from Falcon (Paignton, UK) unless stated otherwise.
3.2. Preparation of Grape Seed Extract
Grape seeds were separated from stems and skins manually and washed and dried in the air in a thin layer at ambient temperature for some days. Dried grape seeds were ground to a fine powder with a grinder. To remove fatty matter, it was treated with hexane for 6 hours in a Soxhlet extractor. The extraction of the powder (40 gr) was performed in a Soxhlet extractor for 10 hours with extractants (150 mL), such as ethanol and acetone. The aqueous extract was prepared using steam distillation (1:5 herb/water, in w/v ratio) for 4 hours by a Clevenger device.
3.3. Determination of GSE Antioxidant Capacity by DPPH
The free radical-scavenging capacity of the extracts was assessed using 2,2-diphenyl-1-picrylhydrazyl (DPPH) following the method described by Ahmed et al. with some modifications (16). In brief, 0.1 mM DPPH in ethanol was prepared, and the solution (2.85 mL) was mixed with 0.15 mL of various levels (5, 15, 25, 50, 100, 200, 400, 800, 1000, and 2000 µg/mL) of aqueous, ethanol, and acetone extracts. It was then shaken strenuously and kept at room temperature for 30 minutes, and its absorbance was read at 517 nm by a spectrophotometer.
3.4. Enzymatic Isolation and Culture of SSCs
The Agricultural Institute of Animal Ethics, Care, and Use of Iranian Research Organization for Science and Technology (IROST) approved the research protocol. Testes were collected after the slaughter and transported to a laboratory on ice within 2 hours at 20 - 35°C to isolate SSCs. After washing three times with deionized water and normal physiological saline, they were 10 times sprayed with 70% ethanol. Supplemented with 50 µg/mL gentamicin and then initial tunica albuginea removal, the testes were cut into small pieces (6 - 10 gr) to digest using an enzymatic treatment in two steps, according to Izadyar et al. (17). In the first step of enzymatic digestion, 1 µg/mL hyaluronidase type II, 1 µg/mL trypsin (Inoclon), 5 μg/mL DNase, and 1 µg/mL collagenase were used. A shaker incubator (150 RPM cycle) was used for incubation for 40 minutes at 37°C. After the first step of enzymatic digestion, the cells were washed three times using Dulbecco’s phosphate-buffered saline (DPBS). For the second enzymatic digestion, the pellet was suspended in Dulbecco’s Modified Eagle Medium (DMEM), containing 1 µg/mL hyaluronidase type II, 5 μg/mL DNase, and 1 µg/mL collagenase. Then, incubation was conducted in a shaker incubator (150 RPM cycle) for 40 minutes at 37°C.
3.5. Enrichment of SSCs
The supernatant containing SSCs, myeloid cells, Sertoli cells, and other cells contaminating the seminiferous tubular tissue underwent filtration through nylon mesh filters of 80 and then 60 µm to enrich the SSCs. Then, the filtered cells were added to Petri dishes (60 mm) covered by lectin-bovine serum albumin (BSA), according to Jafarnejad et al. (18), developed by dissolving lectin (5 µg/mL in DPBS) from Datura stramonium agglutinin in DPBS. The dishes were kept for 2 hours at room temperature, followed by washing using BSA (0.6% BSA in DPBS). For coating BSA, the dishes were also held at ambient temperature for another 2 hours. The cultures were incubated for 5 - 6 hours at 37°C in a CO2 incubator (5% CO2 in air). Then, the remaining medium was transferred to a 15-mL tube. The unattached cells in the medium were collected through centrifugation at 1000 rpm for 5 minutes, and the pellet was re-suspended in DMEM.
3.6. Preparation of Feeder Layers
The feeder layers were prepared from the Sertoli cells. The cells were cultured with DMEM treated with 10% fetal bovine serum (FBS), and the cell suspension was placed on lectin-coated dishes followed by incubation for 2 - 3 days at 37°C and 5% CO2 to develop and generate a confluent monolayer. The cells underwent sub-culturing in a culture flask for propagation, following dissociation with 0.25% trypsin-EDTA. After incubation, the non-adhering cells were discarded, and the cells attached to the bottom of the petri dish were Sertoli cells which were treated with mitomycin C (10 µg mL-1) for 3 hours. Washing of the cultures was performed five times using DPBS and DMEM treated with 10% FBS to remove any traces of mitomycin C, and the attached cells were used as feeder cells for SSC culture.
3.7. Mitochondrial Function Assessment
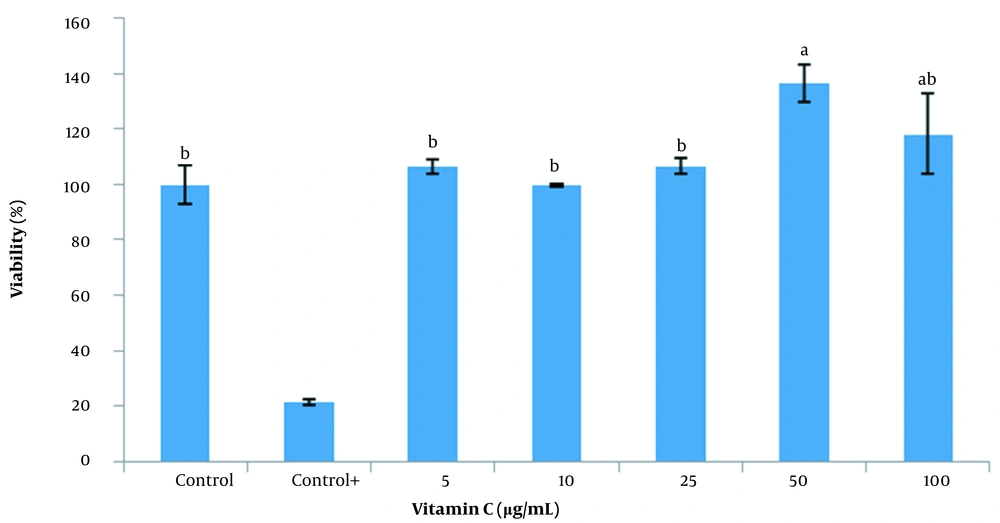
The MTT assay has been most widely used to determine cell viability and cell proliferation (19). It works according to the potential of the mitochondrial dehydrogenases to metabolize MTT to formazan, which occurs for functionally intact mitochondria. In brief, the cells were seeded into plates of 96 wells followed by treatment using different concentrations of aqueous, ethanolic, and acetonic extracts (50, 100, 200, 400, 800, and 1000 µg/mL) of GSE and (5, 10, 25, 50 and 100 µg/mL) vitamin C. After incubation, the cell medium was changed with a fresh one (100 µL/well), and MTT solution (50 μL; 0.5 mg/mL in PBS) was added. After the incubation of the plates for 4 hours at 37°C, generated formazan crystals in DMSO (100-µL) were added to each well. The formazan level was measured using optical density at 570 nm. For all treatments, a coated dish with 96 wells without SSCs was applied to remove the Sertoli cell effect. The control group contained stem cells without treatment. The positive control (control +) group was without using cells.
3.8. Ribonucleic Acid Isolation and Real-time Polymerase Chain Reaction
The TRIzol method was used to isolate total ribonucleic acid. It was reverse-transcribed to cDNA with Maloney murine leukemia virus reverse transcriptase and oligo-DT priming. The changes in the gene expression were studied by real-time polymerase chain reaction (PCR). The amplification procedure included pre-incubation for FastStart polymerase activation at 94°C for 15 minutes and then 40 cycles of denaturation at 94°C for 10 seconds, annealing at 60°C for 15 seconds, and extension at 72°C for 20 seconds. The housekeeping gene β-actin was the endogenous control. Table 1 shows the primers and the lengths of the amplified fragments for real-time PCR. The comparative threshold cycle (∆∆CT) method was used to analyze the data.
| Gene | Annealing Temperature | Amplicon (bp) | Accession Number |
|---|---|---|---|
| BCL2 | 60 | 165 | AY547260.1 |
| F-5'GATGACTTCTCTCGGCGCTA3' | |||
| R-5'GACCCCTCCGAACTCAAAGA3' | |||
| BAX | 60 | 176 | GU731063.1 |
| F-5'GTGAGACCTCTAACCCCACC3' | |||
| R-5'GGTCAGAGGTCATGAGGAGG3' | |||
| β-actin | 60 | 187 | U39357.1 |
| F-5'ACCCAGCACGATGAAGATCA3' | |||
| R-5'GTAACGCAGCTAACAGTCCG3' |
Abbreviations: R, reverse primer; F, forward primer.
3.9. Statistical Analysis
The experiments were performed three times. The values were expressed as mean ± mean squared error. Quantitative data analysis was applied through SPSS software (version 16; IBM, USA). The one-way analysis of variance followed by Duncan’s multiple-range test compared the mean values. The P-values less than 0.05 were regarded as significant.
4. Results
4.1. Antioxidant Capacity of GSE
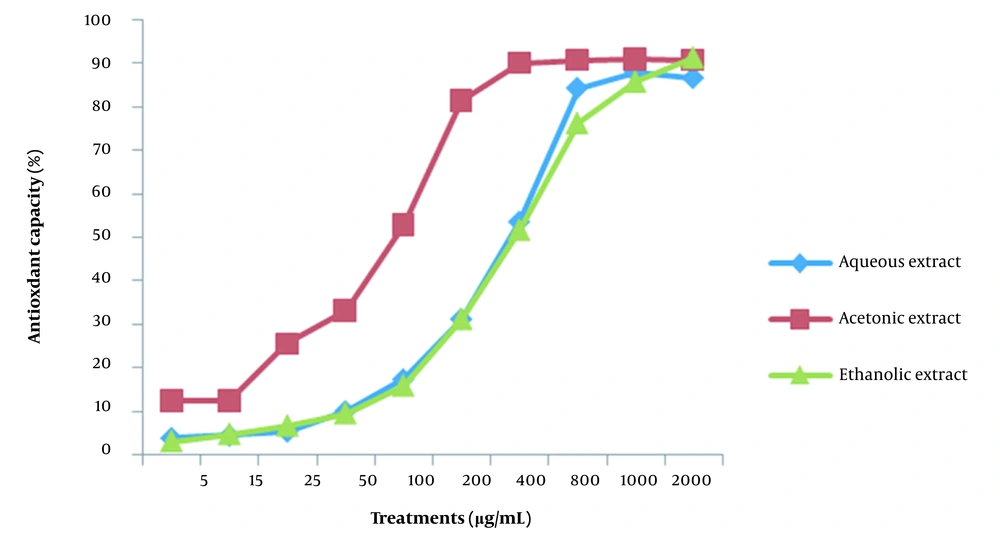
The DPPH method was applied to determine the antioxidant capacity of aqueous, ethanolic, and acetonic extracts of grape seeds. The GSE was used with various concentrations of 5, 15, 25, 50, 100, 200, 400, 800, 1000, and 2000 µg/mL. The extracts of acetonic grape seeds in the concentration of 400 µg/mL showed about 90% antioxidant properties based on the DPPH test. Nevertheless, the aqueous and ethanolic extracts had only 50% of their antioxidant properties at this concentration (Figure 1).
4.2. Effects of GSE and Vitamin C on SSCs’ Viability
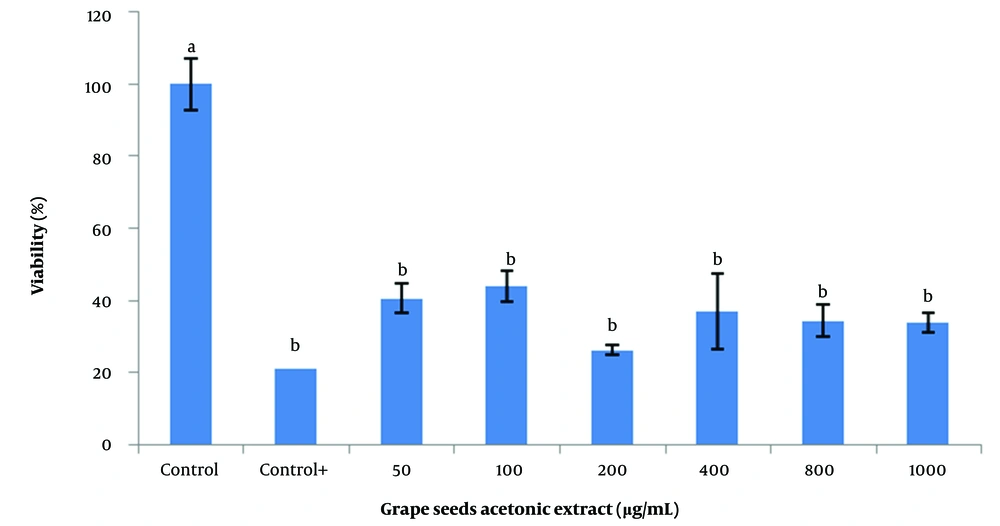
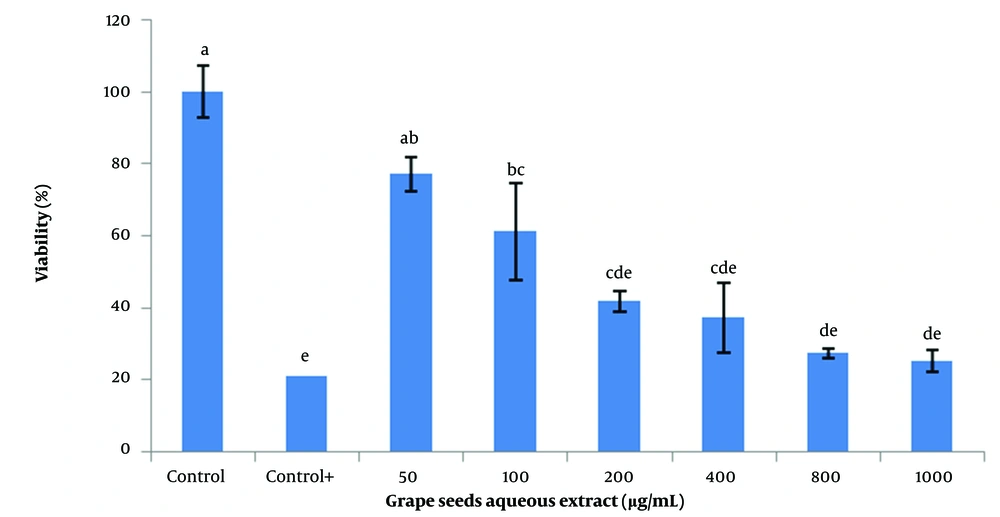
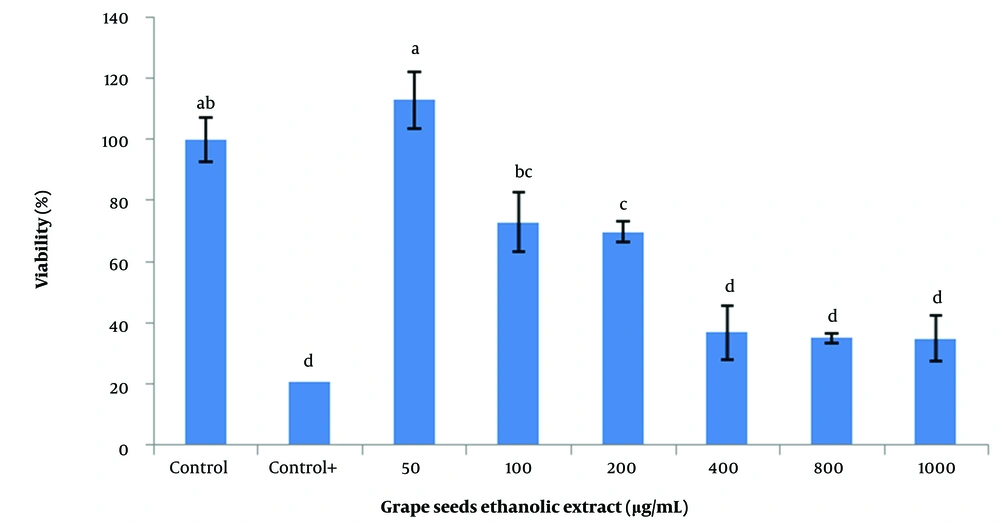
For this purpose, 50, 200, 400, 600, 800, and 1000 µg/mL of each of the aqueous, ethanolic, and acetonic extracts of grape seeds and 5, 10, 25, 50, and 100 µg/mL of vitamin C were used. The findings demonstrated that the acetonic extracts of grape seeds could significantly decrease the viability of SSCs (P < 0.05; Figure 2). Additionally, different concentrations of the aqueous extract negatively affected the viability of SSCs. However, there was no significant difference between the 50 µg/mL aqueous extract and the control (Figure 3). The highest survival rate was obtained from the ethanolic extracts of grape seeds at the concentration of 50 µg/mL, and a significant difference with higher concentrations of ethanolic extracts was obtained (100 to 1000 µg/mL) (P < 0.05; Figure 4).
The viability of SSCs treated with vitamin C (50 µg/mL) was significantly higher (P < 0.05) than the control and 5 - 25 µg/mL of vitamin C groups. Nonetheless, there was no significant difference between the concentrations of vitamin C in the 50 and 100 µg/mL groups (Figure 5).
4.3. Effects of Aqueous, Ethanolic, and Acetonic Extracts of Grape Seeds and Vitamin C on SSCs’ Colony Formation
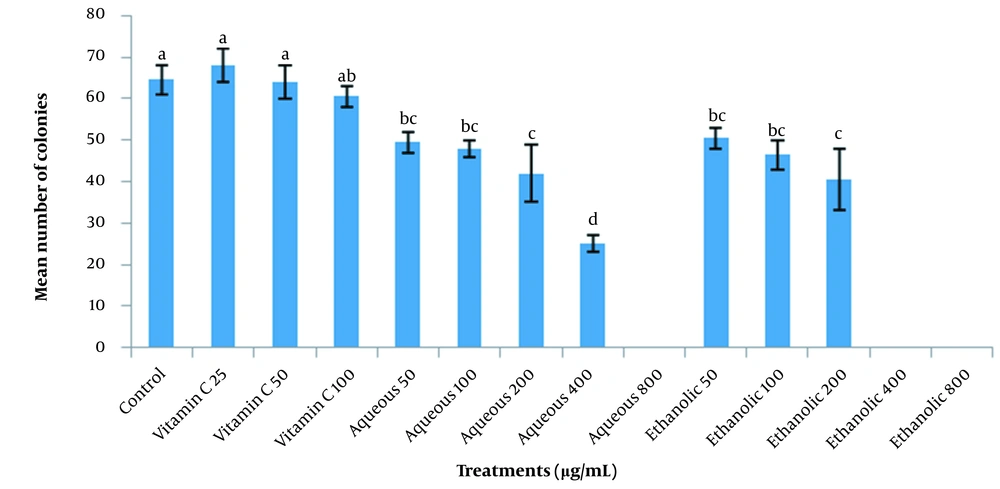
To clarify the effect of aqueous, ethanolic, and acetonic extracts of grape seeds and vitamin C on ovine SSCs’ colony formation, various concentrations of GSE (50, 100, 200, 400, and 800 µg/mL) and vitamin C (25, 50, and 100 µg/mL) were added to the SSCs culture media. The increase in the concentration of the aqueous and ethanolic extracts significantly decreased the colony formation of SSCs (P < 0.05), and no colony formed when acetonic extracts or higher concentrations of aqueous and ethanolic extracts were used (800 and 400 µg/mL for aqueous and ethanolic extracts, respectively). The colony formation of SSCs was not affected by various concentrations of vitamin C, and no significant difference was observed between different concentrations of vitamin C and the control group (Figure 6).
4.4. Expression of Pro-apoptotic and Anti-apoptotic Genes on Ovine SSCs Treated with Effective Doses of Vitamin C and Aqueous and Ethanolic Extracts of Grape Seeds
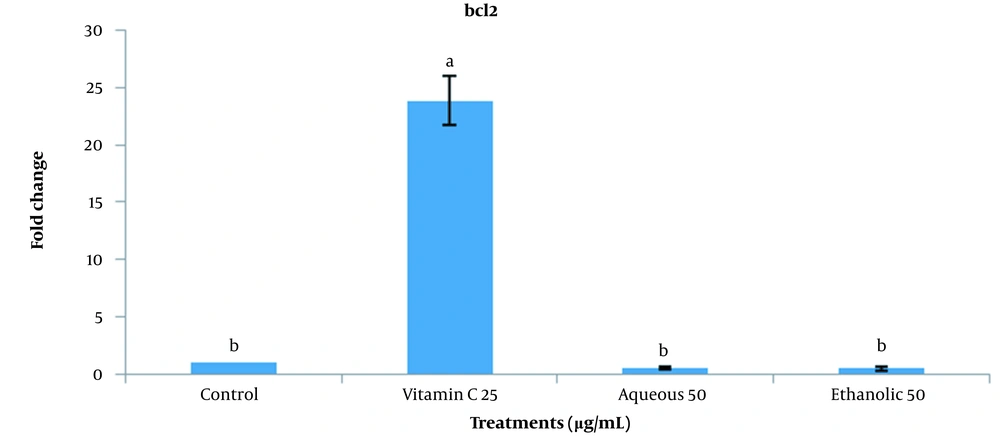
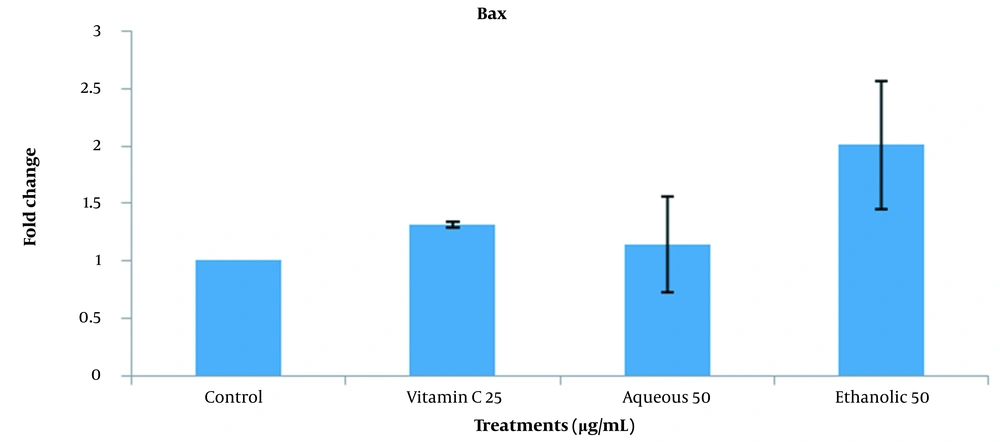
This experiment investigated the expression of BCL2 (anti-apoptotic) and BAX (pro-apoptotic) genes based on the optimum concentrations obtained by colony formation treatments (25 µg/mL vitamin C, 50 µg/mL aqueous extract, and 50 µg/mL ethanolic extract). The expression of the BCL2 gene in vitamin C treatment was significantly higher than the control and aqueous and ethanolic extracts of grape seeds (P < 0.05). The same expression was observed among different extracts and control groups (Figure 7). As shown in Figure 8, no significant difference was observed between different groups in comparing BAX expression.
5. Discussion
The GSE is a rich source of antioxidants (10). Inflammation, cell proliferation, and apoptosis are affected by GSE, not only through antioxidant activity alone but also by gene expression modulation (20). The effect of GSE on male fertility has been reported. Supplementation with GSE restores the spermatogenic process and fertility damage due to toxic heavy metals (21) and decreases oxidative stress-induced effects on spermatogenesis in Swiss mice treated with cadmium (22). A study by Bayatli et al. showed that GSE corrected sperm motility, reduced oxidative damage, and blocked apoptosis (23). The GSE exerted potent effects against oxidative stress and inhibited the generation of free radicals (24). Grape antioxidants can affect the transcription factor nuclear factor kappa B by suppressing its DNA-binding ability to suppress cancer cell invasion (25). The GSE resulted in intracellular ROS accumulation as its main mechanism for growth inhibition, apoptosis, and DNA damage, significantly reversed by the antioxidant N-acetylcysteine (26). Grape polyphenolic compounds play an important role in protecting endothelial cells against oxidative damage and reducing the inactivation of nitric oxide radicals through the modulation of oxidation enzymes, such as NADPH oxidase, superoxide dismutase, and glutathione peroxidase (10).
In this study, the concentration of 50 μg/mL of vitamin C increased the survival rate, and the concentration of 25 μg/mL increased the BCL2 gene expression in SSCs. Culture supplementation with the appropriate vitamin C concentration can protect cells against the ROS damaging effects by inhibiting ROS generation, regulating the expression of apoptosis-associated genes, and promoting stem cell proliferation. Vitamin C can activate anti-apoptotic signals and inhibit pro-apoptotic signals in vitro. Antioxidants at a proper concentration are capable of inhibiting apoptotic signals, activating anti-apoptotic signals, and increasing the SSC population and viability. They prevent SSCs from damaging their cellular structure and apoptosis through a decrease in the level of oxidative stress, as their well-known and innate characteristics (18).
Recent studies also demonstrate that vitamin C is associated with epigenetic reprogramming (27). Suitable vitamin C doses blocked ROS generation and regulated the apoptosis signaling pathway (28). Antioxidants have a possible mediating role in the expression of many factors associated with cell apoptosis, such as Bcl2 (29). However, vitamin C acts as a growth promoter to improve cell proliferation and synthesis of DNA and is protective against ROS’s detrimental effects (15).
5.1. Conclusions
Based on the results of this study, the aqueous, ethanolic, and acetonic extracts of grape seeds should not be used in the culture of SSCs. Nevertheless, the use of 25 - 50 µg/mL of vitamin C is recommended in the culture of SSCs.