1. Background
Studies have shown that regular physical activities, especially aerobic exercises (AE), can prevent the early occurrence of many chronic diseases related to obesity, such as diabetes, cardiovascular diseases, hypertension, dyslipidemia, osteoporosis, and depression (1, 2). A mechanism by which AE exerts its favorable health effects is the improvement of antioxidant defense capacity and the reduction of reactive oxygen species (ROS) (3). Although ROS production increases during intense physical exercise, the improvement and development of the antioxidant defense system are created parallel to this increase, protecting the tissues against the damage caused by ROS (1, 2). However, increased levels of ROS and low activity of antioxidant enzymes, including superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT), are considered indicators of oxidative stress (4, 5). Studies confirm that oxidative stress plays a role in obesity-related complications (6). Recent research shows that ROS can promote muscle adaptations to physical exercise (2, 7). The adaptation process in skeletal muscles is a functional outcome of the intensity and repetition of stimulation, which affects several events and leads to the activation or suppression of specific signaling pathways. Therefore, it regulates gene expression and protein synthesis or degradation (2, 8). The main pathway of cellular protective regulators to endogenous and exogenous stresses caused by ROS is the nuclear factor erythroid 2 (NRF2) transcription factor signaling protein, which can also bind to the inhibitory protein rich in cysteine residues (KEAP1). In fact, the NRF2-KEAP1 signaling pathway is an antistress mechanism that maintains cellular homeostasis (2, 9, 10). Studies report that regular aerobic physical activity in rodents increases NRF2 in skeletal muscles, which can regulate the expression of many antioxidant enzymes (9-11). Another research revealed that Nrf2 not only has an antioxidant effect but also plays an important role in regulating glucose and lipid metabolism in obesity conditions (12).
In fact, when ROS production is not enough or excessive, ROS-mediated signaling and adaptation to physical training are impaired. Evidence shows that Nrf2 deficiency can increase insulin resistance, adipogenesis, and adipocyte differentiation. Overexpression of the Nrf2 gene can also cause insulin resistance under certain conditions (12).
Antioxidant supplements increase the expression of antioxidant enzymes, which include enzymes involved in glutathione synthesis, through regulation of the NRF2/KEAP1 pathway (13, 14). Some studies have demonstrated that these supplements may help with weight loss and recovery after exercise (15-23). Therefore, the use of exogenous antioxidants may help delay muscle fatigue and improve endurance performance (24).
Bitter orange peel (BOP) extract can be considered a suitable source of polyphenols because it contains different types of flavonoids with different concentrations. Polyphenols are the most abundant antioxidants in the human diet. Evidence shows that polyphenol supplements have a great ability to positively affect redox homeostasis and improve the physiological and physical functions of skeletal muscles. The benefits of these compounds are due to their antioxidant and anti-inflammatory properties, their impact on transcription factors, and regulation of the activity of enzymes that adjust the expression of proteins (25). Evidence also emphasizes the essential role of polyphenols in strengthening the antioxidant defense system and stimulating the expression of antioxidant enzymes through NRF2 signaling pathways (26). In fact, polyphenols activate Nrf2 and not only inhibit ROS production but also degrade Keap1 and regulate the Nrf2-KEAP1 signaling pathway (27). The results of a study showed that citrus flavonoid therapy improves vascular function by reducing circulating inflammatory biomarkers and stimulating nitric oxide production. In this way, blood flow to active muscles increases, and fatigue-related metabolites are quickly removed, improving exercise tolerance and muscle recovery mechanisms (13).
Bitter orange peel also contains an alkaloid phenylethylamine (Citrus aurantium L), which is rich in p-synephrine (28). P-synephrine has an adrenergic effect and helps regulate blood glucose, as well as insulin and triglyceride balance. Therefore, using p-synephrine or products containing p-synephrine, along with low-to-moderate-intensity exercise, can help with weight loss (29). Recently, Gutierrez-Hellin and Del Coso showed that p-synephrine can increase fat utilization during submaximal AE. Therefore, this supplement has become a widely used substance to reduce body fat levels (30). Research indicates that citrus flavonoids can control caloric intake versus consumption and regulate lipid metabolism, and their use as a safe and natural alternative to treat obesity is currently under investigation (31).
In addition, antioxidant supplements at high doses can have dual effects on inflammation. In this way, antioxidant levels can be improved through the consumption of exogenous antioxidant supplements. Vitamin C and polyphenols sometimes act as pro-oxidants and can reduce the hormetic response to endurance exercise (32). Some studies also report that supplements reduce adaptation after physical activity (33-36). Therefore, there is a need to measure the levels of oxidative stress and specific food supplements consumed by athletes based on the type, dose, and duration of supplement use.
2. Objectives
The effect of AE and BOP extract on the state of oxidative stress biomarkers of skeletal muscle tissue is not known; therefore, this study aimed to investigate the effect of AE and BOP extract on oxidative biomarkers and the Nrf2-Keap1 signaling pathway in the quadriceps of male rats fed high-fat food.
3. Methods
3.1. Feeding the High-Fat Food
A normal diet in the form of pellets was procured from Behparvar Company (Karaj, Iran) for laboratory animals. The normal food contains crude protein of 20.50 - 19.50%, fat 3.5 - 4.5%, fiber 4 - 4.5%, calcium 0.95 - 1%, phosphorus 0.65 - 0.7%, salt 0.5 - 0.55%, lysine 1.15%, methionine 0.33%, threonine 0.72%, tryptophan 0.25%, and energy (mJkg-1) 16.16 - 17 (37). For obesity induction, 5% cholesterol, 20% palm oil, and 0.25% cholic acid were added to the standard food as a high-fat diet (HFD) (38). The HFD was administered to all the [except for the control-normal diet (ND) group].
3.2. Animals
In the preclinical trial, 30 adults male Wistar rats, 20 weeks old, weighing approximately 300 to 350 g, were purchased from the Pasteur Institute of Iran (Tehran, Iran). After the rats were familiarized with the laboratory environment, they were randomly divided into 5 groups: (1) control (CO)-ND, (2) CO-HFD, (3) HFD-AE, (4) HFD-receiving BOP extract, and (5) HFD-AE-BOP.
3.3. Aerobic Exercise Program
For familiarization with training, the rats in the desired groups were trained to run on a treadmill for rodents at a speed of 9 m/min for 20 minutes a day (including 5 minutes for warming up, 10 minutes of the main training at the mentioned speed, and 5 minutes to cool down) and were placed there for 5 days. After the familiarization period, moderate-intensity training was performed for 4 weeks, 5 days a week. On the first day of training, the rats were trained for 10 minutes on a treadmill at a speed of 16 meters per minute (m/min). During the test period, the speed of the treadmill was gradually increased from 16 to 26 m/min. To start the training, they warmed up for 5 minutes at a speed of 7 m/min and cooled down for 5 minutes at a speed of 5 m/min after the main training.
3.4. Bitter Orange Peel Extract
The supplement used in this research was BOP extract with a special formulation prepared by the Medicinal Plants Research Institute (Karaj, Iran). The supplement prepared in a liquid form was dissolved in distilled water and applied by the gavage method at a dose of 60 mg/kg body weight of rats for 4 weeks, 5 times a week.
3.5. Weighing and Tissue Removal
Forty-eight hours after the end of the AE period and taking the BOP extract supplement, all the rats were fasted for 8 - 10 hours and weighed before the tissue removal. Rats in all the studied groups were anesthetized using ketamine-xylazine (100 mg/kg) and underwent surgery after complete anesthesia to investigate the oxidative changes in the quadriceps tissue. The tissue of the quadriceps was isolated for further measurements, cleaned by washing with phosphate-buffered saline (PBS), and placed inside a coded 2 mL microtube. The microtube was transferred into a nitrogen tank and then kept in a freezer at -80°C until cell analysis.
3.6. Statistical Method
All the data were reported using standard deviation (SD) and mean. Via a split-plot analysis of variance, the main effect of AE, the main effect of BOP, and the interaction of AE and BOP (AE-BOP) on the outcomes were tested for the independent groups. To determine the effect of HFD on the outcomes, t-tests for the independent groups, ND and HFD groups were compared. Then, the difference between the AE-BOP, AE, and BOP groups, and the difference between AE and BOP groups, were compared pairwise using a one-way analysis of variance. When a significant difference was observed, Bonferroni’s post-hoc test was used. The significance level for all the tests was P = 0.05.
4. Results
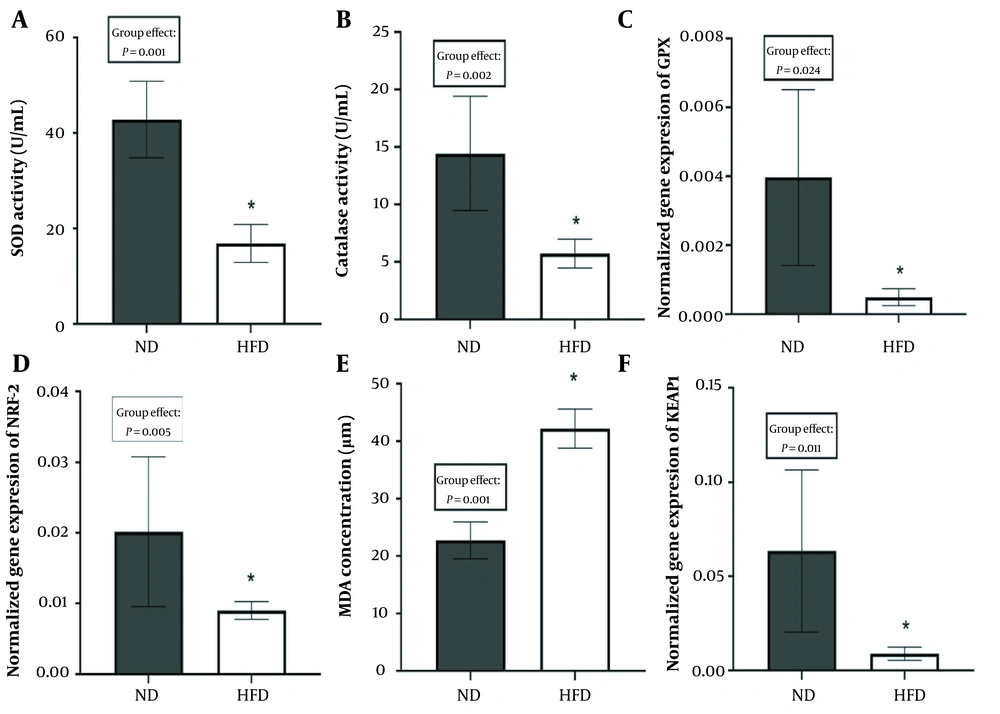
High-fat diet significantly reduced the activity of SOD (P = 0.001), CAT (P = 0.002), GPX (P = 0.024), and NRF2 (P = 0.005) and significantly increased the MDA concentration (P = 0.001) and KEAP1 gene expression (P = 0.011; Figure 1A-F).
Changes in superoxide dismutase (SOD) and catalase enzymes, MDA concentration, and gene expression of glutathione peroxidase (GPX), nuclear factor erythroid 2 (NRF2), and KEAP1 in high-fat diet (HFD) and normal diet (ND) feeding. * P < 0.05, significantly different between ND and HFD. Values are means ± standard deviation, n = 6 independent experiments.
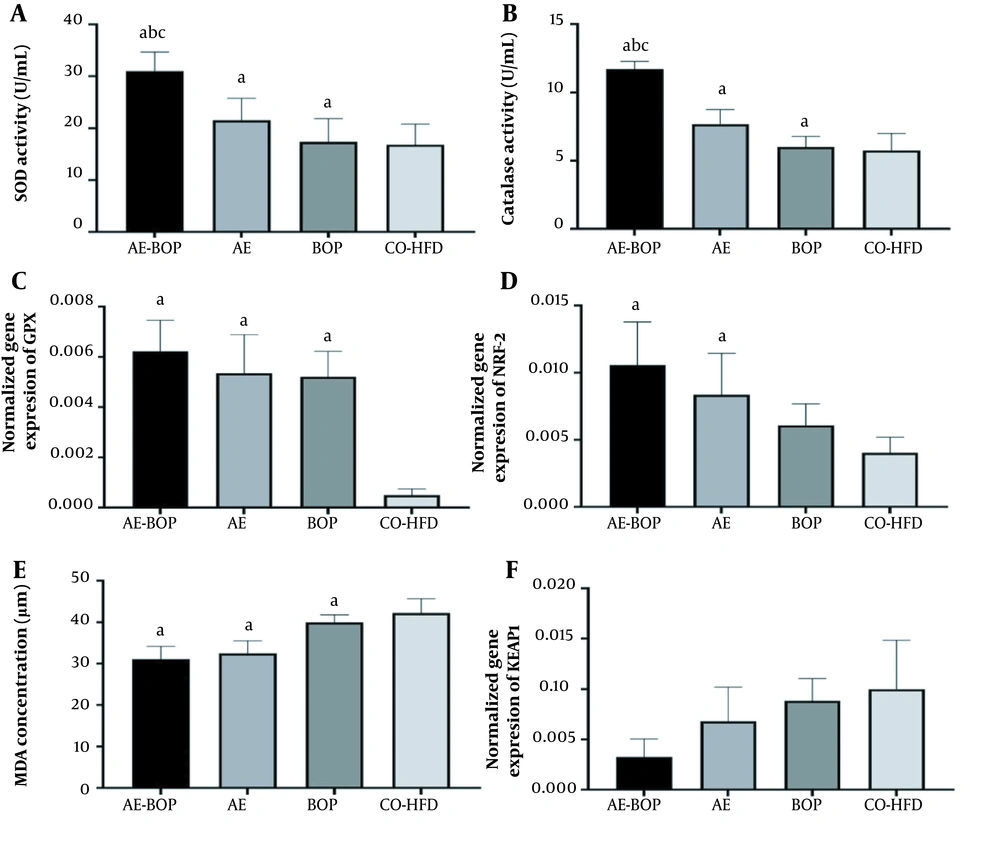
Aerobic exercise significantly increased the SOD enzyme activity of the quadriceps (F = 30.00, P = 0.001, η = 0.600). Bitter orange peel had a significant effect on the SOD enzyme activity of the quadriceps (F = 9.00, P = 0.007, η = 0.310). The AE-BOP significantly affected the SOD enzyme activity of the quadriceps (F = 7.17, P = 0.014, η = 0.264). Aerobic exercise-bitter orange peel also had a significant effect on the SOD activity of the quadriceps. This shows that these two interventions strengthened each other's effect on the activity of this enzyme. The level of SOD enzyme activity in the AE-BOP group was significantly higher than in the AE (P = 0.004), BOP (P = 0.001), and CO-HFD groups (P = 0.000). However, no significant difference was observed between AE and BOP groups (P = 0.571; Figure 2A).
The effect of aerobic exercise (AE), bitter orange peel (BOP), and AE-BOP on antioxidant enzymes, MDA concentration, and gene expressions of glutathione peroxidase (GPX), nuclear factor erythroid 2 (NRF2), and KEAP-1 in high-fat diet (HFD) feeding. a: Significant difference compared to control (CO)-HFD. b: Significant difference compared to AE. c: Significant difference compared to BOP, P < 0.05. Values are mean ± standard deviation, n = 6 independent experiments.
The CAT enzyme activity of quadriceps significantly increased in AE (F = 97.06, P = 0.001, η = 0.829), BOP (F = 30.42, P = 0.001, η = 0.603), and AE-BOP (F = 23.55, P = 0.001, η = 0.541) groups. The CAT enzyme activity in the AE-BOP group was significantly higher than in the AE (P = 0.001), BOP (P = 0.001) and CO-HFD (P = 0.001) groups. The CAT enzyme activity in the AE group was significantly higher than in the BOP group (P = 0.037; Figure 2B).
The GPX gene expression of quadriceps significantly increased in AE (F = 12.23, P = 0.002, η = 0.380), BOP (F = 10.46, P = 0.004, η = 0.343), and AE-BOP (F = 4.78, P = 0.041, η = 0.193) groups. The expression of the GPX gene in the AE-BOP group was significantly higher than in the CO-HFD group (P = 0.001). No significant difference was observed between the AE-BOP (P = 1.000) and BOP groups (P = 1.000). Besides, no significant difference was found between AE and BOP groups (P = 1.000; Figure 2C).
The MDA concentration of quadriceps significantly decreased in the AE (F = 33.90, P = 0.001, η = 0.629), BOP (F = 10.54, P = 0.004, η = 0.345), and AE-BOP (F = 4.50, P = 0.047, η = 0.184) groups. The concentration of MDA in the AE-BOP group was significantly lower than in the CO-HFD group (P = 0.001). However, there was no significant difference between the AE-BOP group and the AE group (P = 1.000) or the BOP group (P = 0.099). Moreover, no significant difference was observed between AE and BOP groups (P = 0.502; Figure 2D).
Aerobic exercise significantly increased the NRF-2 gene expression in the quadriceps (F = 7.56, P = 0.012, η = 0.274), while the BOP (F = 2.09, P = 0.163, η = 0.095) and the AE-BOP (F = 0.007, P = 0.935, η = 0.001) did not have a significant effect on NRF-2 gene expression in the quadriceps. No significant difference was found for the AE-BOP group with AE (P = 1.000) or BOP groups (P = 354). Furthermore, no significant difference was observed between AE and BOP groups (P = 1.000; Figure 2E).
Aerobic exercise (F = 3.46, P = 0.077, η = 0.148), BOP (F = 2.92, P = 0.103, η = 0.128), and AE-BOP did not have any significant effect on the KEAP1 gene expression in the quadriceps (F = 0.011, P = 0.917, η = 0.001; Figure 2F).
5. Discussion
It is well-known that eating high-fat food causes obesity and has negative effects on energy metabolism and protein synthesis in skeletal muscles. In this study, HFD decreased the NRF2 gene expression and increased the KEAP1 gene expression and oxidative stress. The results revealed that the average activity of SOD, CAT enzyme, and GPX gene expression in AE, BOP, and AE-BOP groups increased after 4 weeks. These results are consistent with those of previous studies confirming the positive effect of AE on oxidative stress biomarkers (15, 17, 20, 39-43). Zhang et al. concluded that exhaustive exercise with lemon peel extract supplementation significantly increases SOD and CAT levels (15).
Kheyrdeh et al. investigated the effect of 8 weeks of high-intensity interval training (HIIT) and consumption of citrus extracts on oxidative stress and antioxidant levels of soleus muscle in aged rats. The results showed that HIIT increased GPX and decreased protein carbonyl. The consumption of citrus extract and the interaction between HIIT and citrus extract increased GPX and decreased MDA and carbonyl protein in the soleus muscle tissue of old rats. Finally, HIIT and citrus supplement consumption separately and synergistically had a beneficial effect on reducing oxidative stress and increasing antioxidant activity (44).
In contrast, the research results indicated that supplementation with different antioxidants during endurance training reduces SOD gene expression in rodents (33). Zhou et al. also showed that high-dose astaxanthin supplementation, combined with moderate-intensity swimming activity in rats, reduced CAT and GPX levels in plasma or muscle compared to the control group (35). Meier et al. concluded that supplementing with different antioxidants in rodents during endurance training reduces the GPX gene expression (33). The difference in the results of the studies may be justified based on the difference in the type of activity and supplement, methodological differences in measuring the activity of antioxidant enzymes, and diversity in the types of muscle fibers studied.
The results of the present research demonstrated that the average concentration of MDA in the AE, BOP, and AE-BOP groups significantly decreased after 4 weeks of training and taking the supplements. The reduction of MDA in this study showed that aerobic physical activity can be effective in decreasing skeletal muscle lipid peroxidation in HFD conditions. These results are consistent with those of previous studies (15, 18, 19, 35).
Davaran et al. concluded that AE and capsaicin supplements decreased MDA concentration in HFD rats. Therefore, AE and capsaicin can be used as suitable alternative treatments for obesity and the related inflammatory effects of oxidation (18). Zhang et al. found that endurance exercise, along with lemon peel extract supplementation, significantly lowers MDA levels (15).
Comparing the average activity of the NRF2 gene in the studied groups after 4 weeks of AE and taking the BOP extract showed that the average activity of the NRF2 gene increased in the AE and AE-BOP groups, but the average activity of the KEAP1 gene in these groups did not significantly decrease. However, if the duration of the AE period and consumption of the BOP extract were longer, a significant difference may have been observed compared to the control group. van Iersel et al. reported that antioxidant supplements increase the expression of antioxidant enzymes by raising the expression of the NRF2 gene and decreasing the expression of the KEAP1 gene (13). Chen et al. concluded that curcumin supplementation, by modulating the NRF2-KEAP1 signaling pathway, significantly increased the endurance of rats in the resistance swimming test and lowered exercise-induced oxidative stress. Moreover, curcumin increased the activity of SOD, CAT, and GPX enzymes by activating NRF2 signaling (22). Rahimi et al. found that the herbal supplement, along with endurance training, significantly improved oxidative stress in diabetic rats by raising the expression of the NRF2 gene and decreasing the expression of the KEAP1 gene (21). In contrast, Zhou et al. showed that high-dose astaxanthin supplementation, along with moderate-intensity swimming activity, decreased the level of NRF2 in the gastrocnemius muscle and decreased the gene expression of NRF2-dependent enzymes in the hearts of rats (35).
The results of our research revealed that consuming BOP extract for 4 weeks has no significant effect on NRF2 and KEAP1 gene activity in the quadriceps of HFD male rats. A reason for the nonsignificant difference between the group receiving the BOP extract and the control group may be the short period of supplement consumption (4 weeks). In this regard, Gao et al. showed that after 20 weeks of Qing brick tea consumption, the NRF2 signaling pathway and downstream antioxidant factors increased in the skeletal muscle of mice (45). Also, another study concluded that Q10 supplementation increased the NRF2 gene expression in heart, muscle, and liver tissues after 6 weeks of exercise (46).
Finally, the results of the cited research may depend on the level of physical fitness, type, intensity, and duration of the exercise performed, and the type of supplement and duration of its use.
5.1. Conclusions
In this study, consuming HFD developed oxidative stress in the skeletal muscle tissue. By regulating the NRF2-KEAP1 signaling pathway, AE and BOP extract reduced the oxidative stress caused by HFD. Therefore, it is recommended that these two interventions be used when HFD is consumed.