1. Background
Polycystic ovary syndrome (PCOS), first described in 1935 as a combination of hirsutism, amenorrhea, enlarged cystic ovaries, obesity, and infertility, affects a range of women of reproductive age (1). Polycystic ovary syndrome, a common endocrinopathy, primarily affects women of childbearing age and often begins in adolescence (2). The basic symptoms of PCOS include irregular menstruation, defects in ovulation, infertility, hirsutism, and acne (3). Additionally, there is an increased prevalence of metabolic disorders such as type 2 diabetes mellitus, impaired glucose tolerance, and insulin resistance (IR) among patients with PCOS (4, 5).
The precise etiology of PCOS is poorly understood but appears to involve familial background, genetic predisposition, and environmental factors participate in the occurrence and development of the disease (6). It is not surprising that lifestyle. Lifestyle factors, including diet, food quality, and low exercise, directly affect the risk of PCOS in susceptible individuals (7) by promoting metabolic disorders like IR and type 2 diabetes mellitus. Although genome-wide studies have identified some markers, these show low predictive values (8). Factors such as intrauterine defects, high maternal hormones, and weight gain in women are also likely to contribute to PCOS conditions (9). Thus, it is not possible to focus on a single gene as the primary candidate responsible for the development of PCOS, leading major studies to consider the heterogeneous basis of the condition (6).
Vitamin D, converted into 1,25-dihydroxycholecalciferol in the liver and kidney, regulates calcium and phosphate homeostasis, which in turn adjusts insulin secretion from β-cells (10). The vitamin D receptor (VDR), a key functional arm of vitamin D, forms a heterodimer with the retinoid X receptor (RXR) in many organs and tissues (6). Evidence suggests that VDR may be involved in metabolic diseases (11).
Vitamin D receptor as a transcription factor regulates the expression of genes especially those that participate in the metabolism of glucose by an unrevealed mechanism, to date (10).
The VDR gene located on chromosome 12q13.11 contains 12 exons and encodes a ligand-dependent transcription factor in the steroid/thyroid hormone receptor superfamily (12).
Polymorphisms in the VDR gene may affect the conformation of the receptor as well as the interaction between vitamin D and VDR leading to a decrease in absorption of vitamin D (13). Recent findings have suggested that VDR variations were also associated with the risk of PCOS as well as the severity of the phenotype (12, 14).
2. Objectives
In this study, we aimed to investigate whether VDR gene polymorphism rs2228570T > C is associated with the risk of PCOS in a southeast population of Iran. rs2228570C/T variation occurs in the first exon of VDR mRNA and interrupts the initiator codon. In recent years, the correlation of rs2228570T > C with the risk of PCOS has been analyzed in different ethnicities (12).
3. Methods
3.1. Study Design and Subjects
In this study, 116 women clinically diagnosed with PCOS based on the Rotterdam ESHR/ASRM consensus criteria (2003) were included as the case group. The inclusion criteria consisted of clinical and/or biochemical hyperandrogenism, oligo-anovulation, and polycystic ovary, with the morphology of polycystic ovary under the guidance of Ultrasonography and patients should have at least two of these items (15). These subjects were referred to the Molood Infertility Center at Ali-Ibn-Abi Taleb Hospital, Zahedan, Iran, between August 2018 and September 2019. The control group comprised 130 unrelated women with no history of disorders and regular menstrual cycles. These women had no complaints of hirsutism, acne, alopecia, or endocrinopathy. All subjects were informed about the study and its risks.
3.2. VD3 Levels
All subjects were referred to the biochemical laboratory of Ali-Ibn-Abi Taleb Hospital, where VD3 levels were measured using an ELISA technique (PishtazTeb company, Iran).
3.3. Genotyping
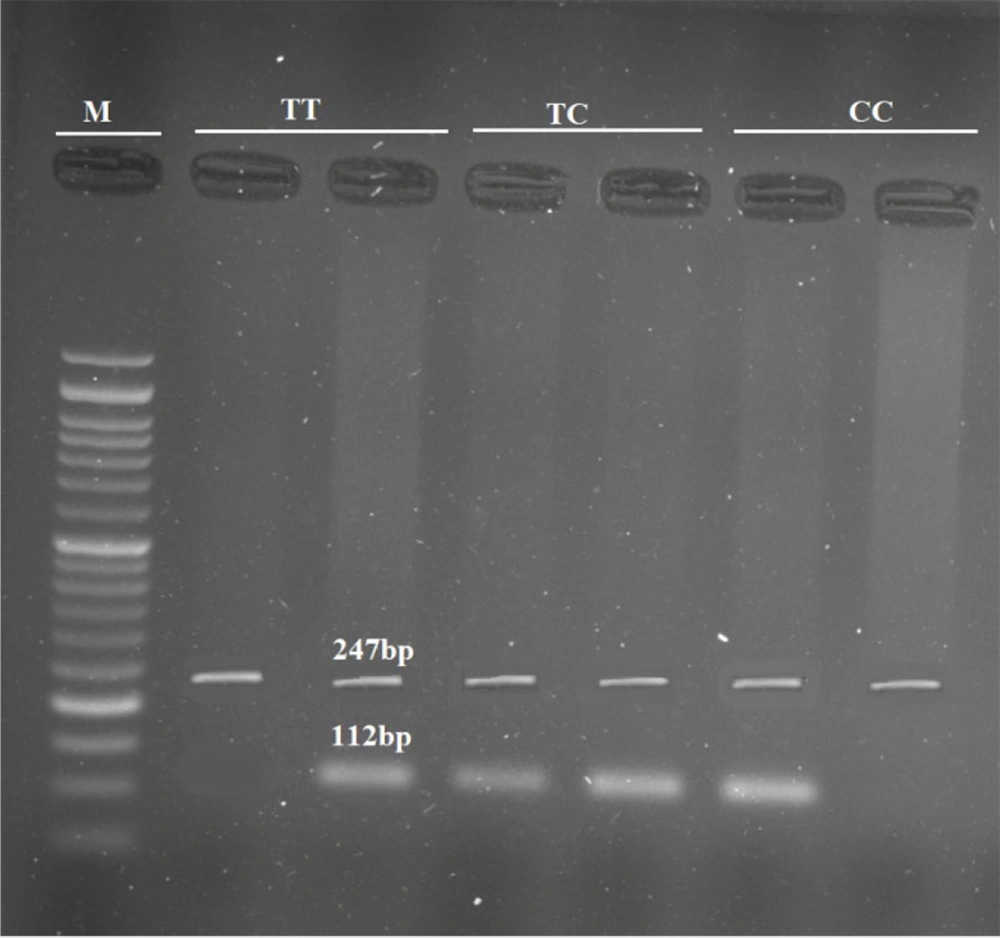
Blood samples were collected from all participants using EDTA-covered tubes. The standard salting-out method was used to extract DNA (16). Genotyping of rs2228570T > C was performed using the amplification-refractory mutation system polymerase chain reaction (ARMS PCR) method. In this technique, each tube contained three primers: Two common primers for amplifying the internal control, and one allele-specific primer for detecting the presence of either allele. The 247 bp internal control fragment was amplified using 5’-GGTCTCCACACACCCCACAGATCCG-3’ as the forward primer and 5’-AGGCCTGGGCCCTGGGGAGAT-3’ as the reverse primer. Additionally, the flanking sequence 5’-GGCCTGCTTGCTGTTCTTACAGGTAT-3’ served as the T-allele-specific primer, and 5’-GGCCTGCTTGCTGTTCTTACAGGTAC-3’ served as the C-allele-specific primer, producing a 112 bp fragment in combination with the forward primer (Figure 1). PCR was performed in an Applied Biosystems VeritiTM Thermal Cycler with 30 cycles of denaturation at 95°C for 30 seconds, annealing at 63°C for 30 seconds, and extension at 74°C for 30 seconds, followed by a final extension at 74°C for 5 minutes. A pre-denaturation step at 95°C for 5 minutes was applied before the ramp cycles. For each sample, a reaction tube contained 10 µL of Taq DNA polymerase 2 × master mix (Parstus Biotech company, Iran), 1 µL of each primer (10 pm/µL) (GenFanavaran Biotech company, Iran), 0.8 µL of extracted DNA, and up to 20 µL of deionized water. The PCR products were subjected to 2% agarose gel electrophoresis and visualized using a UV transilluminator system.
3.4. Statistical Analysis
Statistical analysis was performed using SPSS V.22 for Windows (IBM Corporation, Armonk, NY, USA). Quantitative data were described as mean ± standard deviation for parametric data. Qualitative data were analyzed using numbers and percentages. The Student's t-test and one-way ANOVA were used to compare parametric quantitative data. Genotypic and allelic differences between the two groups were compared using the chi-square (χ2) test, odds ratios (ORs), and 95% confidence intervals.
3.5. In Silico Analysis
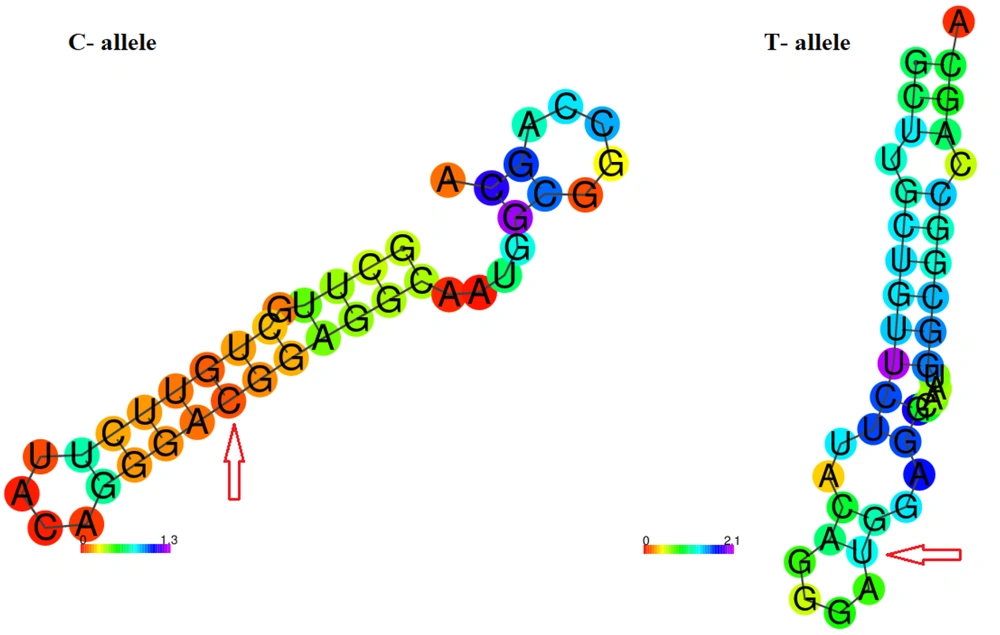
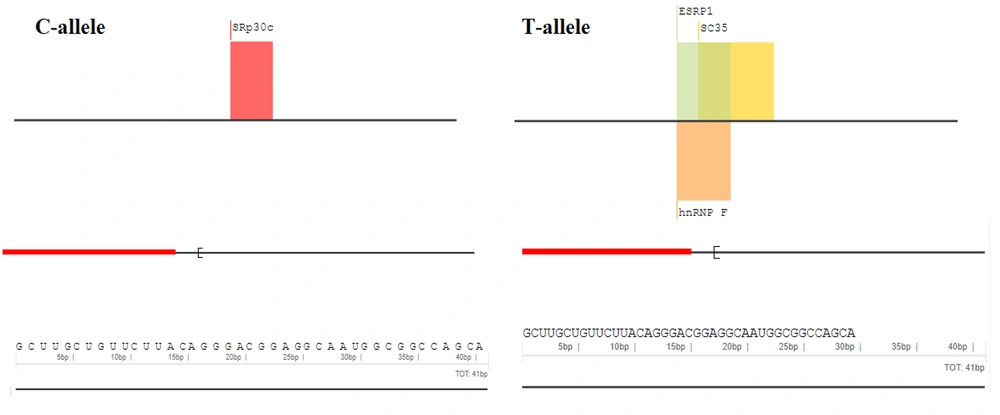
To determine whether rs2228570T > C affects VDR gene products, bioinformatics analyses were performed. The RNAfold server was used to explore the impact of the variant on the secondary structure of the VDR transcript (17) RNAfold introduces a thermodynamic ensemble value in which more negative ones are more stable. SpliceAid2 server was employed to detect the effect of rs2228570T > C on the splicing site patterns (18). Finally, the hypothesis implying the potential of rs2228570T > C for meaningful structural changing was analyzed by the SNAP server which is a server for predicting the functional effects of variants (19).
4. Results
The demographic features of participants were analyzed using the Student's t-test. A P-value under 0.05 was considered statistically significant. There were no significant differences in age and BMI between the two groups. The mean age in the case and control groups was 26.03 ± 4.91 and 27.44 ± 5.35, respectively (P = 0.082) (Table 1). The BMI in women with PCOS and healthy women was 23.73 ± 4.06 and 23.53 ± 4.02, respectively (P = 0.559). A significant decrease in serum vitamin D levels was observed in PCOS cases compared to healthy women (P = 0.001), with levels of 17.92 ± 12.98 and 22.61 ± 13.40, respectively (Table 1).
| Variables | (n ± SD) | P-Value | |
|---|---|---|---|
| PCOS (n = 112) | Controls (n = 150) | ||
| Age | 26.03 ± 4.91 | 27.44 ± 5.35 | 0.082 |
| BMI | 23.73 ± 4.06 | 23.53 ± 4.02 | 0.559 |
| VD3 | 17.92 ± 12.98 | 22.61 ± 13.40 | 0.001 |
z Abbreviations: BMI, Body Mass Index; VD3, cholecalciferol; PCOS, polycystic ovary syndrome; SD, standard deviation.
a P < 0.05 was considered statistically significant.
Table 2 shows the frequency of different genotypes in the studied population. Genotyping analysis revealed that the T-allele significantly decreased the risk of PCOS (OR = 0.65, 95% CI = 0.49-0.95, P = 0.026). Similarly, the TT genotype versus the CC genotype had a protective role against PCOS. The CT genotype versus the CC genotype reduced the risk of PCOS (OR = 0.31, 95% CI = 0.11 - 0.86, P = 0.021). ANOVA showed a statistically significant difference in VD3 levels among genotypes in PCOS women (P = 0.030) (Table 3). VD3 levels were significantly lower in the TT genotype compared to the CC genotype (P = 0.036). However, there was no significant difference in VD3 levels between CC vs. CT and CT vs. TT. There was a significant decrease in VD3 levels in the CC + CT genotype compared to the TT genotype in PCOS subjects (P = 0.001) (Table 4).
| Rs2228570C > T | PCOS | Control | OR (95%CI) | P-Value |
|---|---|---|---|---|
| Codominant | ||||
| CC | 54 (46.5) | 45 (34.6) | 1 [reference] | |
| CT | 56 (48.3) | 69 (53.1) | 0.68 (0.40 - 1.15) | 0.147 |
| TT | 6 (5.2) | 16 (12.3) | 0.31 (0.11 - 0.86) | 0.021 |
| Allele | ||||
| C | 164 (70.7) | 159 (61.2) | 1 [reference] | |
| T | 68 (29.3) | 101 (38.8) | 0.65 (0.49 - 0.95) | 0.026 |
| Dominant | ||||
| CC | 54 (46.6) | 45 (34.6) | 1 [reference] | |
| TT + CT | 62 (53.4) | 85 (65.4) | 0.61 (0.36 - 1.02) | 0.076 |
| Recessive | ||||
| CC + CT | 110 (94.8) | 114 (87.7) | 1 [reference] | |
| TT | 6 (5.2) | 16 (123) | 0.39 (0.15 - 1.03) | 0.083 |
| Over-dominant | ||||
| CC + TT | 60 (51.7) | 61 (46.9) | 1 [reference] | |
| TC | 56 (48.3) | 69 (53.1) | 0.83 (0.50 - 1.36) | 0.533 |
| Additive | ||||
| CC | 54 (46.5) | 45 (34.6) | 1 [reference] | |
| CT | 56 (48.3) | 69 (53.1) | 0.68 (0.40 - 1.15) | 0.147 |
| TT | 6 (5.2) | 16 (12.3) | 0.46 (0.17 - 1.26) | 0.193 |
z Abbreviations: CI, confidence interval; OR, odds ratio; PCOS, polycystic ovary syndrome.
a P < 0.05 was considered statistically significant.
b Values are presented as No. (%).
| Parameter Evaluated | Case Genotypes | Test of Significance | Within Groups Significant | ||
|---|---|---|---|---|---|
| CC | TC | TT | |||
| VD3 | 20.05 ± 10.83 | 17.17 ± 14.74 | 5.73 ± 2.71 | F: 3.62, P: 0.030 | P1: 0.496, P2: 0.115, P3: 0.036 |
a VD3, cholecalciferol; P1, the difference between CC & TC; P2, the difference between TC & TT; P3, the difference between CC & TT; F, One Way ANOVA test.
b Parameters described as mean ± SD.
The related sequences of VDR genes, including transcript and amino acid sequences, were obtained from the National Center for Bioinformatics Information (NCBI) database. A 40-nt flanking region containing rs2228570T > C was selected and introduced to the RNAfold server. The RNAfold server results showed that the C-allele had more negative free energy compared to the T-allele, with thermodynamic ensemble values of -13.34 kcal/mol and -12.31 kcal/mol, respectively (Figure 2). The SpliceAid2 server revealed that the C-allele of rs2228570 creates a de novo binding sequence for the SRp30c splicing factor, while the T-allele creates three new sites for ESRP1, SC35, and hnRNPF, and disrupts the SRp30c site (Figure 3). Finally, the results of the SNAP server depicted that the T > C exchange is not likely to have a significant effect on the structure of the VDR protein. SNAP server predicted rs2228570T > C as a neutral exchange so this variant does not appear to change the structure and function of the VDR protein (Figure 4).
5. Discussion
Polycystic ovary syndrome, distinguished by prolonged lack of ovulation and hyperandrogenism, is one of the most common causes of infertility and dysmenorrhea in reproductive women (20). Due to difficulties in diagnosis, the approaches to diagnosing and managing PCOS vary among physicians and specialties, resulting in different reported prevalence rates in the literature (8). In diagnostic approaches, it is critical to exclude hormone malignancies such as congenital adrenal hyperplasia, androgen-secreting tumors, and Cushing's syndrome due to their similar manifestations with PCOS (6).
In the current study, we found that the T-allele of rs2228570 in PCOS subjects was less frequent compared to healthy subjects and had a protective role, decreasing the risk of PCOS in the studied population. Moreover, the TT genotype versus the CC genotype significantly decreased the risk of disease. VD3 levels showed a significant decrease in PCOS subjects with the TT genotype. In silico analysis showed that the C-allele of rs2228570 is more stable compared to the T-allele. Additionally, our analysis revealed that C and T alleles create new target sites for different splicing factors such as SRp30c, ESRP1, SC35, and hnRNP. Moreover, rs2228570T > C does not appear to affect the structure of the VDR protein.
Vitamin D, resulting from sun exposure or dietary intake, is metabolized to 1,25-dihydroxyvitamin D3 (1,25(OH)2D) in the liver and kidney. Vitamin D, along with parathyroid hormone, controls calcium homeostasis (21). Evidence suggests that vitamin D plays a role in various physiological functions in humans, including reproduction, sex hormone synthesis, and the insulin metabolic pathway (22-24). Vitamin D deficiency has been associated with some comorbidities of PCOS, including hyperandrogenism and cardiovascular disease (25, 26). Polycystic ovary syndrome patients are known to have increased levels of IR compared to healthy women (21). Insulin can increase the level of free testosterone by binding to its receptor (27). Vitamin D appears to affect the expression of insulin receptors and is essential for regulating calcium homeostasis, which is vital for insulin secretion from β-cells (28). Vitamin D regulates the transcription of genes sensitive to vitamin D levels and calcium metabolism through VDR, which acts as a transcription factor (29). Additionally, VDR is involved in estrogen metabolism, impacting ovarian function (30).
Vitamin D receptor gene polymorphisms are likely to impact the etiology of PCOS through the insulin signaling pathway. These polymorphisms may also affect PCOS conditions via the parathyroid hormone (PTH)-vitamin D axis (10, 31), as the interaction between vitamin D and VDR regulates PTH secretion and synthesis (12).
Many investigations have revealed the association of single nucleotide polymorphisms with the risk of PCOS. The VDR gene, involved in the insulin signaling pathway, has been identified as a crucial gene in the occurrence of PCOS (32). A meta-analysis by Shi et al. showed that variations in the VDR gene, such as rs7975232 and rs1544410, are associated with the risk of PCOS, but not rs2228570T > C (12). Mahmoudi found that the CC genotype of rs2228570, compared to CT + TT, confers an increased risk for IR and higher serum insulin concentration in PCOS women (33). An association study by Tuncel et al. showed that rs2228570C > T is linked to decreased vitamin D levels in the serum of Turkish patients compared to the common genotype (30). It is believed that the substitution of C with T leads to the formation of a longer, less active form of VDR. Therefore, carriers of the CC genotype may have higher vitamin D levels than CT and TT subjects (30). Other studies have linked rs2228570T > C with chronic kidney disease (CKD), worsened by vitamin D deficiency (34). Shaymaa and Al-Zubaidy showed that the C-allele and CC genotype of rs2228570T > C are correlated with the risk of type 2 diabetes mellitus (35). In early 2020, Hu et al. analyzed the association of rs2228570T > C with the risk of Parkinson’s disease, finding that C carriers of rs2228570T > C are prone to Parkinson’s disease (36).
In the current study, we faced limitations such as sample size. We plan to extend the population size in future studies and investigate the association of more variations in the VDR gene and other related genes, overcoming technical restrictions.
5.1. Conclusions
According to the findings of the current study, we hypothesize that rs2228570C/T has a protective role against the risk of PCOS in our population.