1. Background
Using herbal compounds to treat diseases is an ancient practice, and until the 19th century, the use of natural resources, mainly plants, was one of the primary methods for treating diseases. The expansion of various scientific fields, such as phytochemical drugs and pharmacology, introduced the use of chemicals in the production of antibacterial drugs. However, due to their unnecessary and incorrect use, scientists have been compelled to revisit plant compounds for the treatment of infectious diseases (1, 2).
Plants, particularly those long used in traditional and folk medicine to combat microbial infections, can be valuable sources for developing new antimicrobial drugs (3).
In Sudan, as in many African countries, the majority of people still rely on traditional or folk medicine for disease treatment. This approach is an integral part of an informal healthcare system, with roots in Islamic and West African medicine (4).
Nanotechnology has gained significant popularity over the last decade due to its important applications and foundations in various scientific fields (5). Among metal nanoparticles, silver and gold nanoparticles, and among metal oxide nanoparticles, titanium dioxide nanoparticles are commonly used in medical devices, electronics, and the food industry due to their unique properties (6, 7).
Historically, nanoparticles have been synthesized only through physical and chemical methods, which are typically expensive and require high temperatures and pressures. Additionally, these methods can be toxic and harmful to the environment and living organisms due to the production of toxic chemicals. Recent developments highlight the important role of biological systems (green methods) in the synthesis of metal nanoparticles, presenting a viable alternative to traditional methods. The low-cost green methods are simple, environmentally friendly, and capable of large-scale synthesis (8, 9).
Nanoparticles are known for their non-toxic and biocompatible nature, making them highly suitable for a range of biomedical applications. These include anticancer (10), anti-inflammatory (11), and antimicrobial properties, as well as targeted drug delivery (12). They also exhibit capabilities in wound healing and biological imaging (13, 14).
Nanoparticles can be synthesized using various methods, including chemical, physical, and biosynthesis techniques, each offering a wide array of properties and applications. While plant-based synthesis of ZnO-NPs has been documented, there is still limited literature on their diverse biological properties, such as antimicrobial, antilarvicidal, protein kinase, and anticancer activities.
Sour tea (Hibiscus sabdariffa L.), a member of the Malvaceae family, is a small tropical annual shrub native to Africa and also found in Southeast Asia and Central America (15, 16). Locally known as Karkede, H. sabdariffa is well-regarded internationally. Various parts of H. sabdariffa are utilized in traditional medicine across numerous countries, including those in Africa, India, Mexico, Brazil, China, and Iran (17).
The leaves, when consumed as a vegetable, possess diuretic, antiseptic, digestive, purgative, sedative, and astringent properties (18, 19). Although the seeds are less commonly mentioned in traditional medicine compared to other parts of the plant, they are smoked and consumed as food and traditionally used as a demulcent, laxative, and tonic (20).
2. Objectives
The purpose of this study is to investigate the synthesis of zinc nanoparticles using the extract of the sour tea plant and to explore its antimicrobial properties.
3. Methods
Mix 5 grams of powder prepared from the sour tea plant with 100 cc of deionized water. Heat the mixture on the stove until it reaches boiling temperature and maintain it for 15 minutes. After it cools, strain it using Whatman No. 1 filter paper and use the resulting aqueous extract for subsequent tests. The extract should be stored at 4°C.
3.1. Synthesis of Nanoparticles
10 cc of the prepared extract was mixed with 90 cc of a 1 mM zinc salt solution, and the mixture was placed on a magnetic stirrer at laboratory temperature for 24 hours. To observe color changes, the absorbance of the solution was measured using a spectrophotometer in the range of 300 - 700 nm. The prepared nanoparticle solution was then centrifuged at 1200 rpm for 15 minutes, and the supernatant was discarded.
3.2. Vegetative Electron Microscope
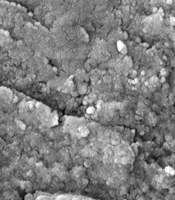
The shape and size of silver oxide nanoparticles were examined using scanning electron microscopy (SEM). To do this, 15 microliters of the zinc nanoparticle solution were applied to specialized SEM grids. After drying, the antibacterial properties of the nanoparticles were assessed.
4. Results
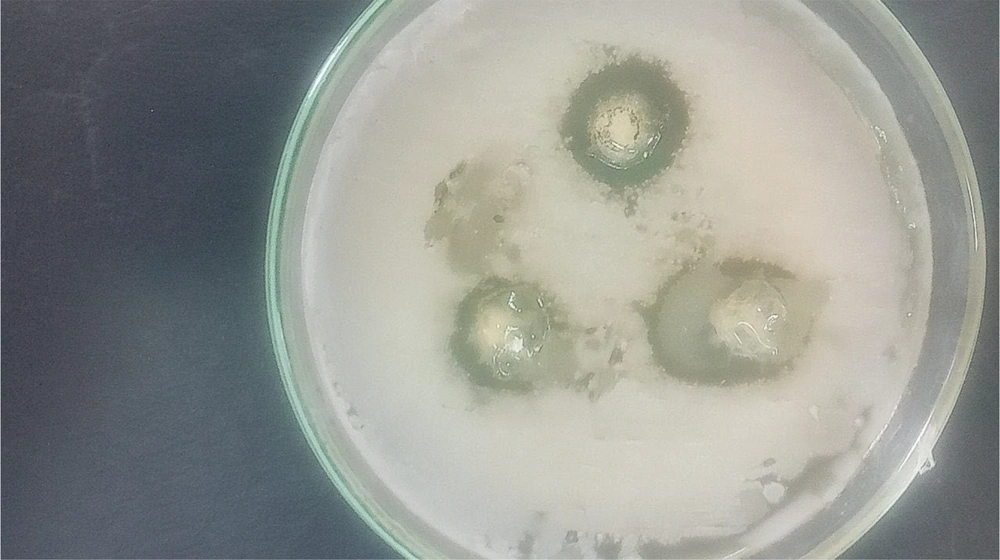
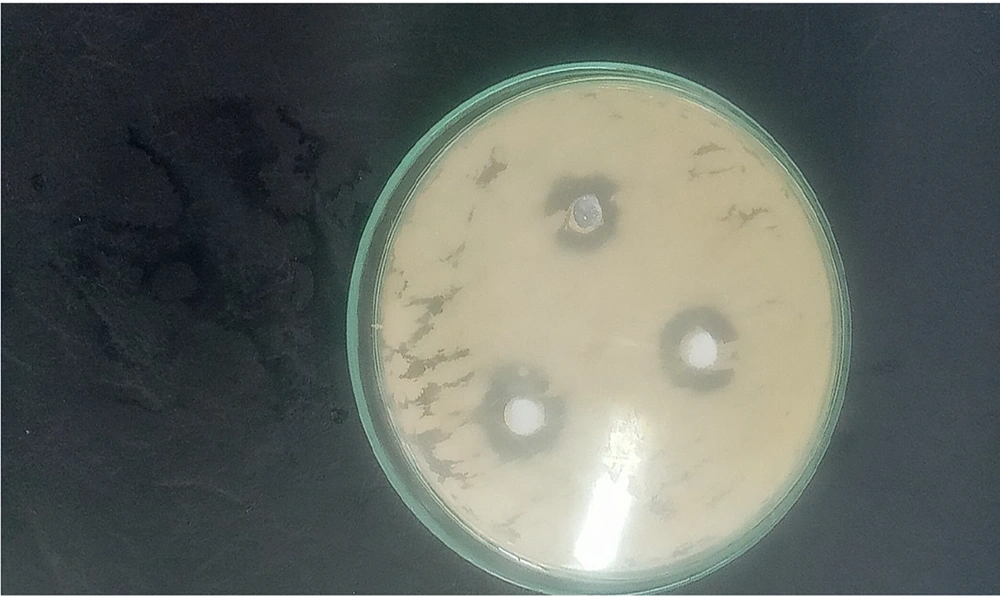
The results of this study indicated that the largest diameter of the inhibition zone was observed at a concentration of 1024 μg/mL against Listeria monocytogenes bacteria (Figure 1), while the smallest inhibition zone was noted for Aeromonas hydrophila bacteria. At a concentration of 512 μg/mL, the diameter of the inhibition zone was 10 mm against two strains of Vibrio cholerae and Escherichia coli bacteria (Figure 2 and Table 1). The image of the synthesized nanoparticles is shown in Figure 3.
| Variables | 1024 µg/mL | 512 µg/mL | 256 µg/mL |
|---|---|---|---|
| Vibrio cholerae | 14 | 10 | 5 |
| Escherichia coli | 15 | 10 | 5 |
| Listeria monocytogenes | 20 | 15 | 6 |
| Aeromonas hydrophila | 4 | 3 | 1 |
5. Discussion
In the study by Tabatabai Yazdi et al., the antibacterial effects of sour tea extracts were examined in vitro against several antibiotic-resistant pathogenic bacteria. The results revealed that E.coli showed resistance to penicillin (75.9%), erythromycin (58.3%), tetracycline (Tet) (56.9%), and cefixime (37%), while Staphylococcus aureus exhibited resistance to penicillin (83%), cefixime (80%), erythromycin (55.6%), and Tet (26.1%). This study demonstrated the beneficial effect of the ethanolic extract of H.sabdariffa on antibiotic-resistant strains of E.coli and S.aureus, with the minimum inhibitory concentration (MIC) of the extract being 16 mg/mL for E.coli and 4 mg/mL for S.aureus (21).
In the study by Rashidi et al., the antioxidant and antimicrobial effects of ethanolic extracts of sour tea and tea grass were investigated. The antibacterial properties of these extracts against pathogenic bacteria (Salmonella enterica, Bacillus cereus, E.coli, and S.aureus) were assessed using the well diffusion method. Additionally, the antioxidant activity of the extracts was measured using the ABTS method, with results compared to the antioxidant capacity of Trolox. The findings indicated that S.aureus was the most sensitive bacteria to both tea grass and sour tea extracts. There was no significant difference between the two plants regarding their antibacterial properties against this microorganism (22).
In another study, the antibacterial test results indicated that the methanolic extract of H. sabdariffa calyces contained effective antibacterial agents. This extract demonstrated a significant zone of inhibition against all tested gram-negative and gram-positive bacteria, performing as well as or better than gentamicin, and substantially more effective than penicillin, which showed weak or no effect (23).
In a study comparing the antimicrobial effects of aqueous extract of red rose (RE), chlorhexidine (CH), amoxicillin-clavulanic acid (ACA), Tet, and metronidazole (Met) against Streptococcus mutans, S.aureus, and Enterococcus faecalis, it was found that at a dilution of 25 mg/mL, the diameters of the inhibition zones were 9.1 mm for S. mutans, 7.5 mm for S. aureus, and 8 mm for E. faecalis (24).
In another study, the antimicrobial activity of sour tea extract was investigated against Streptococcus mitis and Streptococcus oralis bacteria. The results demonstrated that sour tea extract inhibited 29.1% of S. mitis and 63.23% of S. oralis bacteria (25).
Marquez-Rodriguez et al. explored the in vitro antibacterial activity of the phenolic extract of sour tea and its application in extending the shelf life of beef. The study found that the minimum inhibitory concentrations against E. coli, S. enterica, S. aureus, L. monocytogenes, and B.cereus were 300, 300, 200, 200, and 200 mg/mL, respectively (26).
Anvarinezhad et al. conducted a study on the green synthesis of zinc oxide nanoparticles using clove extract and three different heating methods. They evaluated the properties of the nanoparticles and found that the average crystallite sizes of nanoparticles synthesized using microwave, autoclave, and stirring heater methods were 45, 50, and 52 nm, respectively. The antioxidant properties of the nanoparticles were 89%, 85%, and 80% in inhibiting free radicals, and their methylene blue color removal properties were 79%, 70%, and 66%, respectively. The synthesized nanoparticles also exhibited high antibacterial activity against both E.coli and S.aureus (27).
A study aimed at synthesizing zinc oxide nanoparticles under laboratory conditions evaluated their antimicrobial properties for inhibiting biofilm formation and eradicating Klebsiella pneumoniae biofilm. The synthesized zinc oxide nanoparticles had a circular structure with a size of 30 nm. Biofilm formation by K. pneumoniae was assessed using the microtiter plate method. The anti-biofilm activity and biofilm eradication of the zinc oxide nanoparticles were observed at concentrations of 50 and 500 micrograms/mL, respectively.
Ranjbar et al. conducted a study to synthesize green zinc nanoparticles using the extract of brown algae Sargassum ilicifolium and evaluate their antibacterial properties. The results showed that the formed nanoparticles were spherical and crystalline, with sizes ranging from 15.1 to 27 nm. The antibacterial activity of the biosynthesized zinc oxide nanoparticles was assessed using MIC and MBC methods against S.aureus and E.coli. The results indicated that the biosynthesized zinc nanoparticles exhibited significant antibacterial effects against the tested bacteria (28).
In the study by Soosani et al., which aimed to achieve green and extracellular synthesis of zinc oxide nanoparticles using a cell-free extract from Rhodotorula pacifica NS02, the antimicrobial properties of the nanoparticles were investigated. The results showed that zinc oxide nanoparticles with an average size of 42.6 nm were obtained using the cell-free extract solution. Due to the small size and proper distribution of the nanoparticles, a significant inhibitory effect was observed against the tested clinical bacterial isolates (29).
In a study, zinc nanoparticles were synthesized using the aqueous extract of Limonium pruinosum L. Chaz. TEM images revealed that the green ZnO nanoparticles had a hexagonal/cubic shape with an average size of approximately 41 nm. The results demonstrated that both the synthesized zinc oxide nanoparticles and the plant extract exhibited the highest inhibition zones against E.coli, measuring 29 mm and 31 mm, respectively, followed by C. albicans with inhibition zones of 28 mm and 29 mm (30).
In a study, nanoparticles were synthesized using the aqueous extract of Myristica fragrans fruit. The findings indicated that K.pneumoniae was the most sensitive strain to both the nanoparticles (27 ± 1.73 mm) and the nanoparticles coated with imipenem (26 ± 1.5 mm) (31).
5.1. Conclusions
The results demonstrated that the nanoparticles synthesized from the sour tea medicinal plant exhibit a very high inhibitory effect against fish pathogenic bacteria.