1. Background
Stress is defined as an unpleasant stimulus that disrupts an organism's biological equilibrium. Environmental and emotional factors are among the types of stressors that can trigger this response (1). Adaptive reactions requiring modifications to neural and neuroendocrine signaling pathways are essential for managing stress and maintaining homeostasis. Both physical and psychological stressors activate the hypothalamic-pituitary-adrenal (HPA) axis through various brain circuits. The corticotrophin-releasing hormone (CRH), primarily synthesized in the paraventricular nucleus (PVN) of the hypothalamus, regulates the HPA axis and acts as the final common pathway integrating neuroendocrine stress responses (2). Acute stress typically elevates CRH mRNA levels and its release in both hypothalamic and extra-hypothalamic regions. The CRH plays a central role in orchestrating stress responses, including inhibiting reproductive function and behavior in both sexes in response to various stressors, partly through CRH and related peptides (3).
Melanin-concentrating hormone (MCH) is predominantly expressed in the hypothalamus and exerts its effects through two receptors, MCHR1 and MCHR2 (4). Due to the widespread distribution of MCH and MCHR1 in the brain, the MCHergic system is believed to influence neuronal activity and play a role in various functions, including mood regulation and anxiogenic responses (5). Research by Kim et al. demonstrated that stress induces an increase in MCH synthesis in the lateral hypothalamus, hippocampus, and other emotion-related brain regions (6). Given the high expression of MCHR1 in brain areas associated with emotional control, MCH signaling is thought to modulate depression and anxiety behaviors (7).
Although many medications are available for treating anxiety, challenges such as slow onset, poor absorption, and adverse effects persist. Flavonoids are a key component in many pharmacological and medical treatments. Isoflavones, including formononetin, are a subclass of flavonoids found in soybeans, chickpeas, and red clover. This group of compounds has been extensively studied for its role in managing central nervous system (CNS) disorders, such as anxiety and depression. Isoflavones have been shown to have anti-stress and anti-depressant effects by modulating several mediators (8). Formononetin, a natural isoflavone, exhibits significant anti-inflammatory and neuroprotective properties (9). Studies have also demonstrated its anxiolytic effects in the basolateral amygdala (10). Additionally, formononetin has been identified as a neuroprotective agent against Alzheimer’s disease, ischemia, and other neurological disorders (11).
2. Objectives
Previous studies have shown that formononetin improves depression by upregulating the glucocorticoid receptor, brain-derived neurotrophic factor, and promoting hippocampal neurogenesis (12). To further explore the molecular mechanisms underlying the anti-stress effects of formononetin, this study investigated its impact on hypothalamic Mch and Crh gene expression in a rat model of stress.
3. Methods
3.1. Animals
Male Wistar rats were housed under standard laboratory conditions with a 12-hour light/12-hour dark cycle in a temperature-controlled room (21 ± 2°C), with food and water provided ad libitum.
3.2. Experimental Design
The animals were randomly divided into four groups (n = 5). Group 1 received a saline injection as the control group, while groups 2, 3, and 4 were stressed groups that received saline, 20 µg, or 40 µg of formononetin, respectively. The drugs were injected 30 minutes prior to stress induction via the third cerebral ventricle.
3.3. Surgery and Cannulation
Rats were anesthetized using ketamine/xylazine and positioned in a stereotaxic apparatus. A cannula was implanted for third cerebral ventricle injections at coordinates AP = 0.84 mm, ML = 0, and DV = 6.5 mm, according to the stereotaxic atlas (13, 14). The animals were given one week to recover before being used in the experiment. The injections were administered using a polyethylene tube connected to a Hamilton syringe. After the experiment, the hypothalamus was isolated and stored at -70°C for mRNA analysis.
3.4. Acute Restraint Stress
To induce restraint stress, the rats were placed in a transparent plexiglass tube (5 cm wide and 18 cm long) for 2 hours. Formononetin was administered, and stress was induced 30 minutes after the injection (15).
3.5. Real-time Polymerase Chain Reaction (RT-PCR)
The RT-PCR technique was used to evaluate Mch and Crh gene expression. Total RNA was extracted using the Trizol kit (Qiagen, Germany). For cDNA synthesis, 1 μg of total RNA was used according to the kit instructions. To measure gene expression levels, 1 μg of cDNA was used for the RT-PCR reaction (Biotech Rabbit, Germany), following the instructions of the SYBR Green I Kit (Takara Bio Inc., Japan). The sequences of the primers are listed in Table 1. The gene expression changes were calculated using the 2-ΔΔCT equation.
| Variables | Primers Sequences | Amplified Product |
|---|---|---|
| Crh | 103 bp | |
| Forward | 5′- TGGATCTCACCTTCCACCTTCTG -3′ | |
| Reverse | 5′- CCGATAATCTCCATCAGTTTCCTG -3′ | |
| Mch | 195 bp | |
| Forward | 5′- TCAGAAGGAAGATACCGCAGA -3′ | |
| Reverse | 5′- ACTGCTGGTCCTTTCAGAGC -3′ | |
| GAPDH | 120 bp | |
| Forward | 5′- AAGTTCAACGGCACAGTCAAG -3′ | |
| Reverse | 5′- CATACTCAGCACCAGCATCAC -3′. |
3.6. Statistical Analysis
Data were analyzed using SPSS software, one-way ANOVA, and Tukey's post hoc test. The results are presented as mean ± SEM, and differences were considered statistically significant at P ≤ 0.05.
4. Results
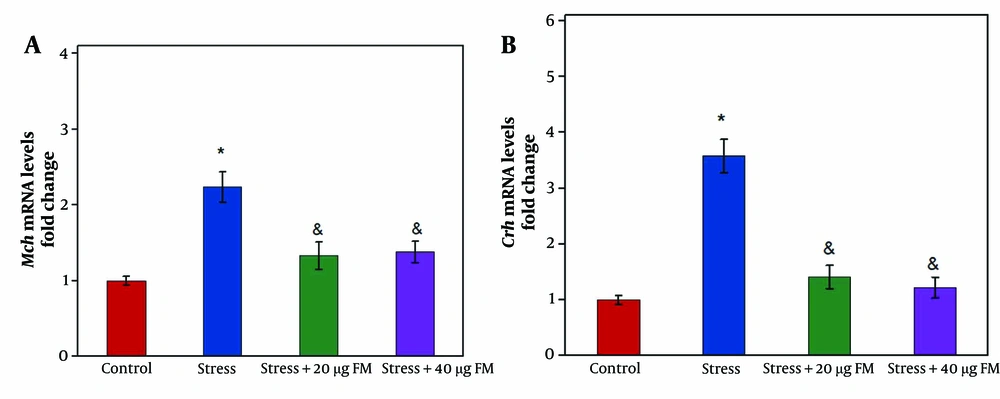
The Mch mRNA level was significantly increased in the stressed rats (group 2) compared to the control group (group 1) (Figure 1A, P ≤ 0.05). However, in rats receiving 20 µg (group 3) or 40 µg (group 4) of formononetin, the Mch mRNA level significantly decreased compared to the stressed rats (Figure 1A, P ≤ 0.05).
Similarly, the Crh mRNA level was significantly elevated in the stressed rats (group 2) compared to the control group (group 1) (P ≤ 0.05). Administration of 20 µg (group 3) or 40 µg (group 4) of formononetin significantly reduced Crh mRNA levels in comparison to the stressed rats (Figure 1B, P ≤ 0.05).
5. Discussion
This study demonstrated that stress increases the expression of the Crh gene, aligning with previous research that shows the activation of the hypothalamic-pituitary-adrenal (HPA) axis in response to stressful situations (2-16). Another study indicated that chronic stress leads to sustained elevation in the activity of CRH neurons in the paraventricular nucleus (PVN) of the hypothalamus (17). These CRH neurons, which control the HPA axis, play a key role in mediating physiological responses to stress. By integrating various stress-related inputs, CRH neurons coordinate the behavioral, endocrine, and immunological responses to stress (18). However, their function is modulated by other neurotransmitters, such as glutamate, dopamine, norepinephrine, GABA, and other neuropeptides (19).
Research on the therapeutic potential of plant-based products for anxiety and depression has accelerated recently. This study aimed to assess the anxiolytic properties of formononetin in rats and explore possible mechanisms of its action. Flavonoids have been shown to benefit the management of neurological diseases like depression and anxiety (10, 11). In rats treated with formononetin, CRH gene expression decreased, supporting previous findings that formononetin helps reduce anxiety in an inflammatory pain model (10).
It has been suggested that noradrenergic and dopaminergic neurons are critical in activating the HPA axis (20, 21). Formononetin exerts an inhibitory effect on norepinephrine and dopamine release (22), suggesting that it may downregulate Crh gene expression by reducing the activity of noradrenergic and dopaminergic neural circuits.
The axons of glutamatergic neurons project onto CRH neurons (23), and glutamate receptors, including NMDARs and AMPARs, are densely expressed on these neurons (24). A study by Zhou and Fang demonstrated that stress induces hyperactivation of the HPA axis by increasing the activity of NMDAR in PVN-CRH neurons (2). Previous studies have also shown that stress elevates the frequency of glutamatergic EPSCs in CRH neurons (25). Thus, blocking glutamatergic NMDAR can downregulate the HPA axis and reduce plasma corticosterone levels in stressed rats (2-18). Tian et al. (2013) found that formononetin protects cortical neurons from NMDA-induced apoptosis (26), and they further established that isoflavones like formononetin can directly bind to estrogen receptors to regulate gene expression via the estrogen response element (11). Wei et al. suggested that estrogen protects against the adverse effects of repeated stress on glutamatergic transmission (27). Therefore, formononetin may reduce HPA axis activity, partly by downregulating glutamatergic neural circuits.
Since the HPA axis and CRH neurons are activated by MCH (28), this study measured Mch mRNA levels in the hypothalamus after an acute stress challenge and formononetin injections. Mch mRNA levels were upregulated in the stressed rats, consistent with studies showing that hyperactivity of hypothalamic MCH neurons influences stressful behaviors (29, 30). Additionally, previous research indicates that chronic stress activates MCH neural pathways in mice (31). Formononetin caused a decrease in hypothalamic Mch gene expression. Wang et al. (10) demonstrated the anxiolytic effects of formononetin. The MCH system plays a role in emotional dysfunction, and MCH receptor antagonists have shown anti-stress and anti-depressive effects. Previous research indicates that hypothalamic MCH neurons receive inputs from glutamatergic neural circuits (32). Sankhe et al. found that deleting Vglut2 from MCH neurons results in anxiolytic responses (32). Furthermore, the anxiogenic effects of glutamate are similar to those of MCH, suggesting that glutamate and MCH may work synergistically to regulate anxiety-like behaviors (32). The anti-glutamatergic action of formononetin may contribute to the downregulation of hypothalamic MCH in stressed rats.
The present study suggests that formononetin may improve stress by downregulating hypothalamic Crh and Mch expression. However, further research is needed to fully understand formononetin’s role in stress management, particularly by investigating its effects on other stress-related genes or proteins such as orexin, neuropeptide Y, neuropeptide S, phoenixin, and calcitonin gene-related peptide in both acute and chronic stress models.
5.1. Conclusions
The results showed that the induction of stress significantly increased the mRNA levels of Crh and Mch. Formononetin exerted inhibitory effects on hypothalamic Crh and Mch gene expression in the stressed rats. This suggests that formononetin may have promising therapeutic potential for anxiety by regulating hypothalamic neural circuits upstream of CRH neurons, such as MCH neurons.