1. Background
Chemotherapy is a common strategy for cancer treatment, defined as the use of chemical agents to halt the growth and destroy cancer cells. However, it not only targets cancer cells but also affects other rapidly dividing cells in the body, such as hair and blood cells, leading to systemic toxicities (1, 2). 5-Fluorouracil (5-FU) is a widely used chemotherapy drug administered for the treatment of various cancer types. It exerts its anti-cancer effects by inhibiting nucleotide thymidylate synthase and incorporating toxic metabolites into DNA and RNA (3, 4).
Treatment of testicular cancer with 5-FU has been associated with a decrease in the weight of male reproductive organs, structural changes in the seminiferous tubule epithelium, and reduced serum testosterone levels (5, 6). Oxidative stress caused by chemotherapy drugs can lead to both temporary and permanent harmful effects on male fertility. Consequently, strategies to mitigate the side effects of chemotherapy drugs have gained significant attention (7).
In recent years, antioxidants have emerged as potential therapeutic interventions due to their ability to combat oxidative stress and prevent its role in cancer development (8, 9). The primary function of antioxidants is to scavenge or neutralize free radicals and inhibit the deleterious downstream effects of reactive oxygen species (ROS).
Taurine (TAU) is one of the most abundant free amino acids, biosynthesized by various mammalian tissues, including the central nervous system, liver, retina, mammary glands, brain, and kidney (10-14). It is found in foods such as meat, seafood, and milk. Taurine plays a crucial role in various cellular processes, including calcium ion regulation, membrane stabilization, immune response, retinal growth, ion transport, and reproduction. It also exhibits antioxidant, anti-inflammatory, hepatoprotective, antidiabetic, antimicrobial, and antitumor properties (15, 16).
2. Objectives
The present study aimed to investigate the potential protective effects of TAU against testicular toxicity induced by 5-FU administration in adult male rats.
3. Methods
3.1. Pharmacological Materials
5-Fluorouracil was manufactured by Ebewe Pharma Ges.m.b.H. Nfg. KG, 4866 Unterach, Austria. Taurine was purchased from Sigma Chemical Co., St. Louis, MO, USA.
3.2. Experimental Design
All experiments were conducted under protocols approved by the Animal Ethics Committee of Shahid Chamran University of Ahvaz, Ahvaz, Iran (EE/1400.2.24.25684/scu.ac.ir). Animal care and use were carried out in accordance with the guidelines of the National Research Council. Thirty-five adult male Wistar rats (6 - 8 weeks old, weighing 200 - 250 g) were obtained from the animal house of Ahvaz Jondishapur University of Medical Sciences (JUMS), Ahvaz, Iran. The rats (three per cage) were housed under controlled conditions: A temperature range of 25 ± 2°C, relative humidity of 55 ± 5%, and a 12-hour light/dark cycle. Food and tap water were provided ad libitum. The animals were acclimatized for two weeks before the experiment. The rats were randomly divided into five groups (n = 7 per group): A control group that received normal saline and four treatment groups injected intraperitoneally (IP) with the following: 20 mg/kg 5-FU, 20 mg/kg 5-FU + 50 mg/kg TAU, 20 mg/kg 5-FU + 100 mg/kg TAU, and 20 mg/kg 5-FU + 200 mg/kg TAU. The treatments were administered daily for two weeks, with TAU given 1 hour before 5-FU administration. At the end of the experiment, the rats were weighed, and blood samples were collected after euthanasia under anesthesia. The testis, ventral prostate, seminal vesicles, and epididymis were removed and weighed. The right testis was fixed in formalin saline for histological analysis, while the left testis was sectioned and stored at -80°C for biochemical assays.
3.3. Endocrinological Analysis
Animals were fasted overnight, and blood samples were collected via cardiac puncture after euthanasia under anesthesia. The blood samples were centrifuged at 4000 g for 5 minutes, and the sera were stored at -30°C. Levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone (T4) were analyzed using radioimmunoassay kits (Monobind Inc., Lake Forest, USA).
3.4. Biochemical Analysis
The left testis was homogenized in ice-cold 0.1 M sodium phosphate buffer (10% w/v) and then centrifuged twice at 10,000 rpm for 15 - 20 minutes at 4°C. The resulting supernatant was used to analyze antioxidant enzyme activity and lipid peroxidation levels. Colorimetric assay kits (Randox Labs Ltd., Ardmore, United Kingdom) were used to measure glutathione peroxidase (GPx) levels and superoxide dismutase (SOD) activity. Malondialdehyde (MDA) levels, as an index of lipid peroxidation, were assayed using the thiobarbituric acid test, measured at 532 nm, and expressed as nmol/mg.
3.5. Histological and Histometrical Analysis
All rats were sacrificed, and their testes were removed and fixed in formalin saline. For histological and histometric analysis, tissue samples were embedded in paraffin, sectioned at 5 - 6 µm, and stained with hematoxylin and eosin. Germinal epithelium height (GEH) and seminiferous tubule diameter (STD) were measured using a microscopic ocular graticule in 90 round or nearly round cross-sections of tubules from each rat.
3.6. Statistical Analysis
SPSS version 16 software (SPSS Inc., Chicago, IL, USA) was used to analyze statistical differences between groups. Analysis of variance (ANOVA), followed by Tukey’s test, was performed. Data are expressed as the mean ± SEM, and a P-value of < 0.05 was considered statistically significant.
4. Results
4.1. Body and Reproductive Organs Weights
The mean body weight of 5-FU-treated rats decreased significantly (P < 0.001) compared to the control group. In the groups receiving 50, 100, and 200 mg/kg of TAU along with 5-FU, body weight increased significantly (P < 0.001) compared to 5-FU-treated rats. However, the mean body weight of the groups receiving 50 and 100 mg/kg of TAU along with 5-FU remained significantly lower (P < 0.001) compared to the control rats. In the group receiving 200 mg/kg of TAU along with 5-FU, the mean body weight showed no significant difference (P = 0.115) compared to the control rats (Table 1).
| Parameters | Control | 5-FU | 5-FU + 50 TAU | 5-FU + 100 TAU | 5-FU + 200 TAU |
|---|---|---|---|---|---|
| Body (g) | 302.4 ± 1.6 | 228.7 ± 1.9 c | 251.4 ± 2.3 c, d | 274.5 ± 2.3 c, d | 293.2 ± 3.5 d |
| Testis (mg) | 1328.9 ± 5.3 | 1001.7 ± 6.4 c | 1248.9 ± 6.2 c, d | 1302.4 ± 5.5 c, d | 1326.3 ± 5.8 d |
| Epididymis (mg) | 536.8 ± 6.1 | 449.1 ± 2.3 c | 459.1 ± 4.1 c, d | 522.8 ± 2.5 d | 526.4 ± 2.0 d |
| Seminal vesicle (mg) | 466.2 ± 8.0 | 450.4 ± 4.9 | 459.2 ± 1.8 | 462.8 ± 6.5 | 461.4 ± 5.0 |
| Ventral prostate (mg) | 203.2 ± 3.6 | 196.0 ± 1.6 | 199.1 ± 2.7 | 198.4 ± 2.7 | 199.8 ± 2.7 |
Abbreviations: 5-FU, 5-fluorouracil; TAU, taurine.
a Data are expressed as mean ± SEM.
b ANOVA followed by Tukey’s test.
c Shows significant difference (P < 0.05) between the 5-FU treated rats and the control group.
d Shows significant difference (P < 0.05) between 5-FU treated rats and the group receiving 5-fluorouracil along with taurine.
The mean testis and epididymis weights in 5-FU-treated rats decreased significantly (P < 0.001) compared to the control group. A significant dose-dependent increase (P < 0.001) in mean testis and epididymis weights was observed in rats that simultaneously received 5-FU and different doses of TAU, compared to the 5-FU-treated group. No statistically significant differences (P > 0.05) were observed between 5-FU-treated rats and the control group in the weights of seminal vesicles and ventral prostates (Table 1).
4.2. Endocrinological Analysis
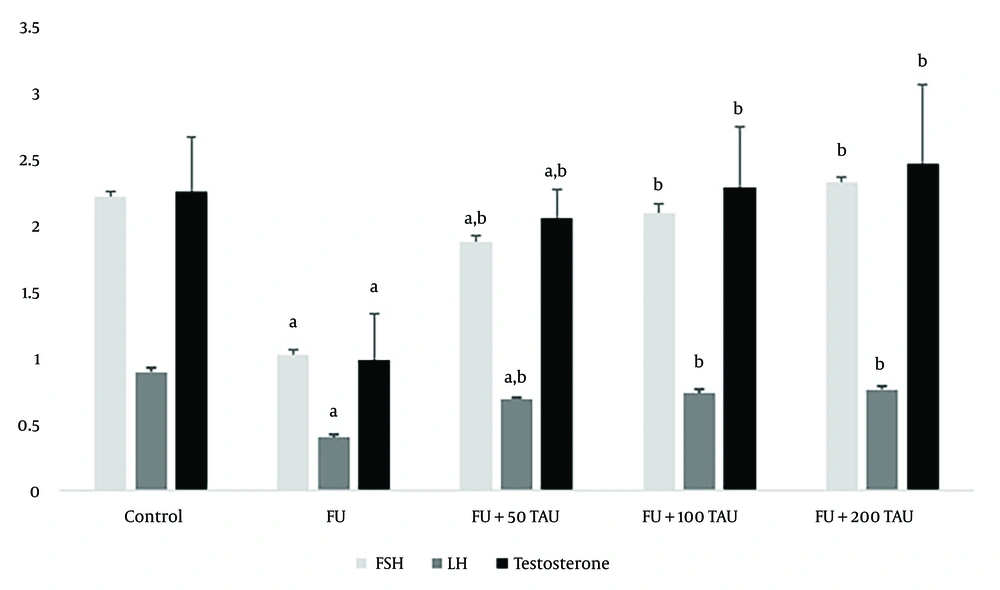
Significant decreases (P < 0.05) were observed in the mean serum concentrations of T4, LH, and FSH in rats treated with 5-FU compared to the control group. In 5-FU-treated rats co-administered with different doses of TAU, significant dose-dependent increases (P < 0.05) were observed in the mean serum levels of T4, LH, and FSH compared to the group receiving 5-FU alone (Figure 1). The levels of T4, LH, and FSH in the groups receiving 100 and 200 mg/kg of TAU along with 5-FU showed no significant differences compared to the control rats (Figure 1).
Mean ± SD of serum follicle-stimulating hormone (FSH), luteinizing hormone (LH) and T4 following the co-administration of 5-fluorouracil (5-FU) and different doses of taurine (TAU) in adult male Wistar rats. a, shows significant difference (P < 0.05) between the 5-FU treated rats and the control group; b, shows significant difference (P < 0.05) between 5-FU treated rats and the group receiving 5-FU along with TAU.
4.3. Biochemical Analysis
In rats treated with 5-FU, significant increases (P < 0.001) in testicular MDA levels and decreases in testicular SOD and GPx activities were observed compared to the control group. In 5-FU-treated rats co-administered with different doses of TAU, significant dose-dependent decreases (P < 0.001) in testicular MDA levels were observed compared to the group receiving 5-FU alone (Table 2). Testicular MDA levels in the groups receiving 100 and 200 mg/kg of TAU along with 5-FU showed no significant differences compared to the control rats (Table 2). Dose-dependent increases in testicular SOD and GPx activities were observed in 5-FU-treated rats co-administered with different doses of TAU compared to the group receiving 5-FU alone (Table 2).
| Parameters | Control | 5-FU | 5-FU + 50 TCU | 5-FU + 100 TCU | 5-FU + 200 TCU |
|---|---|---|---|---|---|
| MDC (nmol/mg protein) | 3.15 ± 0.7 | 10.66 ± 1.3 c | 7.33 ± 0.91 c, d | 5.08 ± 0.99 d | 3.30 ± 0.71 d |
| GPx (U/mg protein) | 7.69 ± 0.37 | 1.84 ± 0.25 c | 4.40 ± 0.56 c,d | 6.14 ± 0.55 d | 5.92 ± 0.53 d |
| SOD (U/mg protein) | 29.01 ± 1.78 | 14.69 ± 1.27 c | 22.76 ± 1.89 c, d | 27.32 ± 0.89 d | 28.26 ± 1.33 d |
Abbreviations: MDA, malondialdehyde; GPx, glutathione peroxidase; SOD, superoxide dismutase; 5-FU, 5-fluorouracil; TAU, taurine.
a Data are expressed as mean ± SEM.
b ANOVA followed by Tukey’s test.
c Shows significant difference (P < 0.05) between the 5-FU treated rats and the control group.
d Shows significant difference (P < 0.05) between 5-FU treated rats and the group receiving 5-FU along with TAU. ANOVA followed by Tukey’s test.
4.4. Histological and Histometrical Analysis
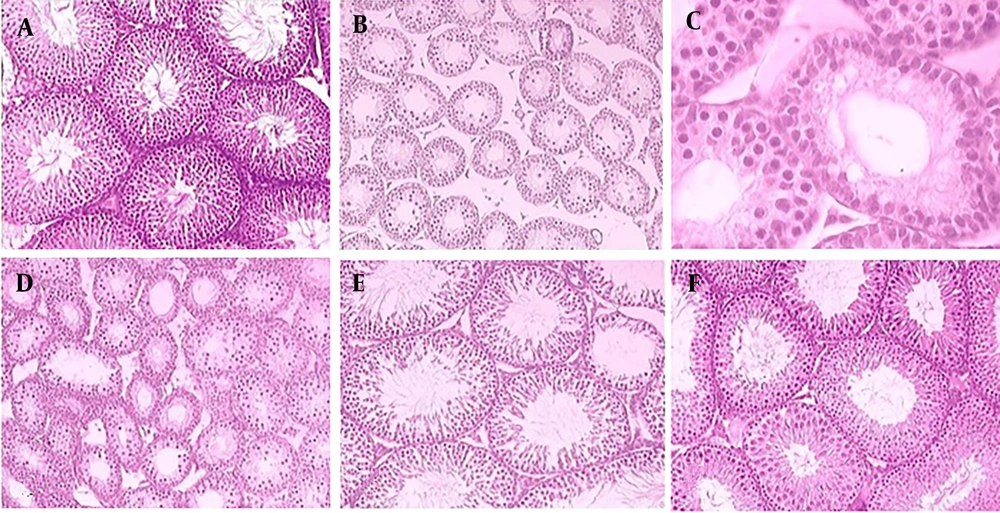
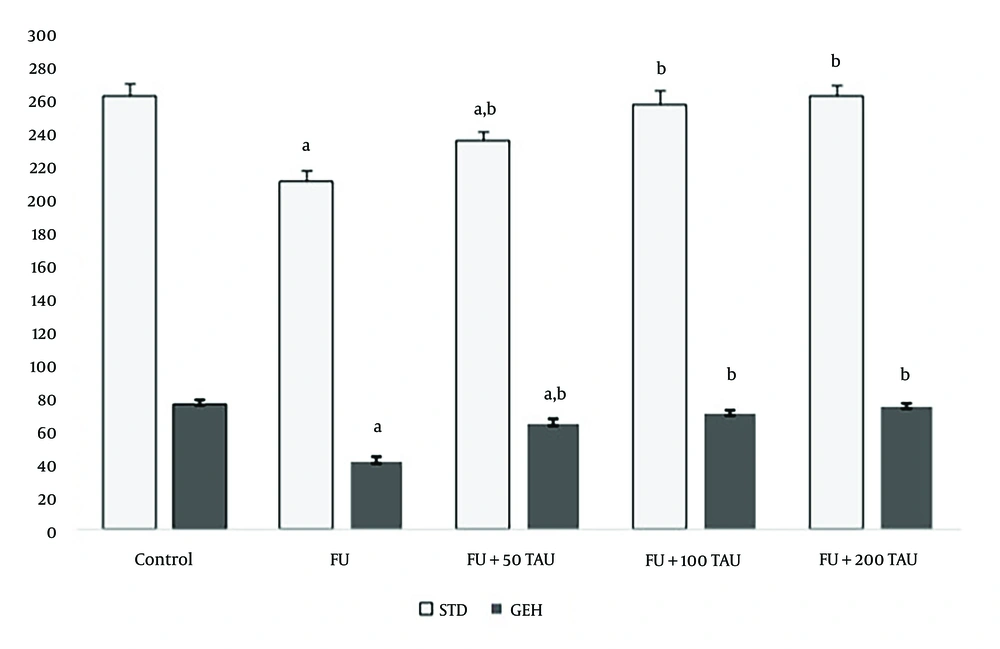
Degenerative alterations in the STD with loss of spermatogenesis, a decrease in GEH, and an increase in tubular lumen were observed in 5-FU-treated rats. Numerous vacuoles were present in the germinal epithelium, and the lumens of some seminiferous tubules lacked spermatozoa due to spermatogenesis arrest in rats receiving 5-FU (Figure 2A - F). Seminiferous tubule diameter and GEH decreased significantly (P < 0.001) in rats exposed to 5-FU compared to the control group. In rats treated with 5-FU and co-administered with different doses of TAU, significant increases (P < 0.001) in STD and GEH were observed compared to 5-FU-treated rats (Figure 3). In the groups receiving 100 and 200 mg/kg of TAU along with 5-FU, the mean STD and GEH showed no significant differences (P = 0.115) compared to the control rats (Figure 3).
Mean ± SD of seminiferous tubules diameters (STD) and germinal epithelium height (GEH) following the co-administration of 5-fluorouracil (5-FU) and different doses of taurine (TAU) in adult male Wistar rats. a, shows significant difference (P < 0.05) between the 5-FU treated rats and the sham group; b, shows significant difference (P < 0.05) between 5-FU treated rats and the group receiving 5-FU along with TAU.
5. Discussion
The use of chemotherapeutic drugs is one of the most common treatments for destroying cancerous cells. However, in addition to their beneficial effects, these drugs also have side effects on healthy body cells. Reducing the harmful effects of chemotherapy drugs while increasing their effectiveness remains a key concern for cancer researchers. In the present study, the effects of TAU were evaluated on testicular toxicity induced by 5-FU in male rats. Our findings indicate that TAU has protective effects on testicular structure and significantly mitigates the testicular oxidative toxicity caused by 5-FU administration.
Several studies have demonstrated that TAU can prevent the potential side effects of chemotherapy drugs on normal cells due to its antioxidant, anti-apoptotic, and anti-inflammatory properties (17, 18). The present study showed that TAU administration increases the activity of GPx and SOD enzymes and decreases lipid peroxidation levels in the testes of 5-FU-treated rats. In this regard, Lee et al. reported that TAU improves GSH levels and enhances the activity of several liver antioxidant enzymes, including GPx, GST, and CAT, in rats subjected to foot-shock-induced stress (19). Aly and Khafagy found that endosulfan increases sperm DNA fragmentation, while TAU administration protects sperm chromatin integrity due to its antioxidant properties (20). Alam et al. demonstrated that TAU supplementation reduces brain, bone, and liver toxicities caused by MTX and TAM (21).
Additionally, Yousef and Aboelwafa reported that TAU, through its antioxidant properties, provides protective effects against nephrotoxicity induced by 5-FU in male rats (22). A study by Noruzi and Zareh concluded that TAU acts as an antioxidant and prevents the consequences of oxidative stress caused by cisplatin (23). Furthermore, Yoshimura et al. showed that UVB radiation decreases moisture and TAU content in the epidermis of mice, but TAU supplementation was able to maintain skin moisture and restore the epidermis (24).
Furthermore, the anti-tumor properties of TAU have been reported in several studies. Tu et al. demonstrated that TAU inhibits the proliferation of A549 human lung cancer cells and the growth of transplanted tumors in mice, enhancing A549 cell apoptosis by increasing BAX protein and Puma levels while decreasing BCL-2 protein levels (25). Zhang et al. reported that TAU induces cellular apoptosis and inhibits cell proliferation in human colon cancer cells (26). Additionally, the protective effects of TAU against the proliferation of prostate cancer cells were reported by Song et al., while Li et al. found that TAU induces apoptosis in cervical cancer cells (27, 28).
5.1. Conclusions
The findings of the present study suggest that TAU ameliorates 5-FU-induced testicular damage in Wistar rats, likely due to its antioxidant properties counteracting oxidative toxicity. Further molecular studies are needed to elucidate the underlying mechanisms of TAU's protective effects.