1. Background
There are about 20 species of the genus Nigella, which is a member of the Ranunculaceae family and is spread from Mediterranean to west Asia (1). Nigella seeds contain important mineral elements, including Potassium as the most abundant element, followed by phosphorus and calcium. Mg, Na, Fe, Zn, Mn, and Cu made up the rest of the matter, in descending order by quantity. These results are in line with those found by Takruri and Dameh (1998) (2). Furthermore, Nigella seeds constitute a rich source of fatty acid esters such as Arachidonic acid, Myristic acid, stearic acid, palmitic acid, oleic acid, linoleic acid, linolenic acid, Dihomolionolenic acid, Eicosenoic acid, Myristoleic acid, Behenic acid, and Eicosadienoic acid (3). The Essential oil of Nigella sativa contains thymol, Thymoquinone (TQ), Dihydrothymoquinone (DHT), ρ-Cymene, Carvacrol, t-Anethole, α-Thujene, α-pinene, β-pinene, Sabinene, β-Myrcene, Limonene, Ethyl hexanoate, γ- terpinene, and Terpinolene; TQ (active ingredients) including compounds that responsible for a major part of the pharmacological effects of Nigella sativa (4).
In addition, many therapeutic effects of NS extracts, such as antioxidant properties (5), anti-inflammatory properties (6), antibacterial properties (7), a stimulatory effect on the immune system (8), its Anti-stress, learning, and spatial memory enhancement properties (9), have been recently recorded in the literature.
Hans Selye defined stress as nonspecific changes caused by function or damage and a state of threatened homeostasis. Stress is basically a mental and bodily reaction against the homeostatic changes (10). Oxidative stress is resulted from reactive oxygen species (ROS); ROS are produced from molecular oxygen during normal cellular metabolism and can be beneficial at low concentrations. However, ROS constitute a class of highly reactive free radicals and oxygen-containing molecules, which include singlet oxygen, hydroxyl radical (OH-), superoxide radical (O2-), hydrogen peroxide (H2O2), and lipid peroxides, which in turn result in continuous damage to lipids, proteins, and DNA (11).
Immobilization stress results in oxidative damage in rats and antioxidant administration effectively inhibits the oxidative damage resulted from stress treatment. With immobilized stress, both plasma and the cerebral cortex lipid peroxidation go up while protection in the plasma against iron-induced oxidation and free radical scavenging, membrane fluidity, and Na+/K+ATPase activity decrease. However, it increases brain monoamine levels and their metabolic rate. Furthermore, the administration of either reduced glutathione or Yizhiyishou, an antioxidant product extracted from medicinal plant, indicated a protective effect against stress-induced stomach bleeding, oxidative damage, and abnormal changes in the antioxidant defenses and monoamine neurotransmitters in the cerebral cortexor in plasma. Based on these observations, Liu et al., (1996), hypothesized that stress may induce the formation of oxidants and induce oxidative damage (12).
2. Materials and Methods
2.1. Plant Materials and Extraction Procedure
They were purchased from a local herb market. Then, the varieties of nigella sativa seeds were confirmed by Herbarium University of Urmia. The seeds of N. sativa were utilized to prepare the extract according to WHO protocol CG-04 (13). For preparation of alcoholic extraction, the seeds were dried, powdered and then subjected to the extraction method for extraction with 96% ethanol, whose residues was filtered and then evaporated to dryness under reduced pressure, which finally yielded about 4.8% of residue.
2.2. Animals
Female Wistar rats weighing about 160 - 200 g were provided by Urmia University breeding colony and kept for a week before being used in a well-ventilated room maintained at 25 ± 1 ºC, 12 h light/12 h dark cycles, while they freely accessed water and standard pellet diet. In this laboratory study, we considered all the ethical principles of how to work with laboratory animals.
2.3. Immobilization Stress
The animals were immobilized with their heads down in supine position, while fixed to a board in an inclined position daily for 2 hrs for the period of 21 days (14).
2.4. Oil Extraction and Preparation of Fatty Acid Mmethyl Esters
The seeds samples were grounded via a ball mill. Lipids were extracted using ethanol (2/1v/v), then, they were centrifuged at 10 g for 5 min, filtered, and finally the solvent was removed on a rotary evaporator at 40ºC. Lipids were extracted with heptane by extractor, lipid extracts were converted into methyl esters via 2 % sulphuric acid (v/v) in methanol and then were extracted by n-hexane. Then, the methyl esters were separated and quantified by gas chromatography and flame ionization detection (Schimadzu GC, 17 Ver. 3) coupled to a glass GC10 software computing recorder. Chromatography was performed with a capillary column (30 m in length and 0.25 mm in diameter, Permabound 25, Machery-Nagel, Germany) and used nitrogen as carrier gas (flow rate, 0.8 mL/min). The temperatures of the column, detector, and injector valve were 130 - 220 ºC and 240- 280 ºC, respectively. Identification was conducted by frequent comparison with authentic standard.
2.5. Ash and Mineral Contents
To get rid of carbon, about 5 g of powdered seed samples were burned in the muffle furnace at 550 °C for about 12 h, whose ashes were dissolved in HCl. The EDTA method and the Spectrophotometric vanadium phosphomolybdate method were used to determine Calcium and the phosphorus content, respectively (15).
2.6. Tissue Samples
After chopping up the tissue into pieces on ice, a 10% (w/v) homogenate was prepared in 10 mM phosphate buffer (pH 7.4) and centrifuged at 13,000 × g for 10 min at 4 °C. The supernatant was utilized to evaluate enzyme activities.
2.7. Catalase (CAT) Enzyme Activities
CAT activity was assayed by the Aebi (1983) method. One unit of CAT activity was determined as the amount of enzyme needed to decompose 1µmol of H2O2 in 1 min. A total of 10% of homogenates of liver and kidney, prepared in 0.9% NaCl in a cuvette containing 1.9 mL of a 50 mM phosphate buffer (pH 7.0), 0.05 mL of tissue supernatant was added. The reaction was started by the addition of 1.0 mL of 30mM H2O2. The decomposition rate of H2O2 was measured by spectrophotometric at 240 nm for 60s. Employing the reaction time (∆t) of the absorbance (A1 and A2), (k): k = (2.3/∆t) (log A1/A2) equation used for compute the rate constant. The catalase activity was indicated on a milligram wet weight as k/mg protein (16).
2.8. Ferric Reducing / Antioxidant Power (FRAP) Assay
The FRAP reagent is made up of 300 mM acetate buffer (3.1 g sodium acetate + 16 ml glacial acetic acid, made up to 1 liter with distilled water; pH = 3.6), 10 mM tripyridyltriazine (TPTZ) in 40 mM HCl and 20 mM FeCl3.6H2O in the ratio of 10:1:1. Briefly, 50 μl of kidney homogenate was added to 1.5 ml freshly prepared and pre-warmed (37 ºC) FRAP reagent in a test tube and incubated at 37 ºC for 10 min. The blue colored complex absorbance was read against reagent blank (1.5 ml FRAP reagent + 50 μl distilled water) at 593 nm. Standard solutions of Fe 2+ in the range of 100 to 1000 mM were prepared from ferrous sulphate (FeSO4.7H2O) in distilled water. The data was expressed as mmol ferric ions reduced to ferrous form per liter (FRAP value). The FRAP assay measures the change in absorbance at 593 nm owing to the formation of a blue colored Fe2+- TPTZ compound from the colorless oxidized Fe3+ form by the action of electron donating antioxidants (17).
2.9. Determination of Lipid Peroxidation in Tissues
Thiobarbituric acid (TBA) reactivity was used to estimate the Lipid peroxidation in the tissue. Considering all these results, the researchers set up the assay procedure for lipid peroxide level in animal tissues as follows: 0.2 ml of 8.1% sodium dodecyl sulfate (SDS), 1.5 ml of 20% acetic acid solution adjusted to pH 3.5 with NaOH, and 1.5 ml of 0.8% aqueous solution of TBA were added to samples less than 0.2 ml of 10% (w/v) tissue homogenate. The mixture was made up to 4.0 ml with distilled water, and then heated in an oil bath at 95°C for 60 min using a glass ball as a condenser. When cooled with tap water, 1.0 ml of distilled water and 5.0 ml of the mixture of n-butanol and pyridine (15: 1, v/v) were added and shaken vigorously. Then it was centrifuged at 4000 rpm for 10 min. 1, 1, 3, 3 -tetramethoxypropane (TMP) was employed as an external standard, and the lipid peroxide level was stated as nmol of MDA. If an assay with a small amount of tissue, such as small organ or biopsied sample is needed, fluorometric measurement (excitation: 515 nm; emission: 553 nm) is applicable instead of spectrophoto metric measurement at 532 nm. When a sample included some amount of biliibin, fluorometric measurement is recommended (18).
3. Results
3.1. Chemical Characteristics of Seeds
The compositions of the Nigella sativa are shown in Table 1. Nigella sativa seeds contain 39.4 ± 3.17, 4.9 ± 0.3% of fat, ash contents, respectively. Mineral composition indicated that calcium is (168 ± 10.9 mg/100g. P < 0.05), phosphorous (73.02 ± 0 mg/100g. P < 0.05). Fatty acid profile is obvious from the results that linoleic, oleic, palmitic, stearic, and linolenic acids were amounting (46.05 ± 1.3, 33.46 ± 1.02, 15.05 ± 0.65, 3.46 ± 0.13, 1.98 ± 0.04%, respectively P < 0.05).
| Composition of Nigella sativa Seeds | |
|---|---|
| Proximate Composition (%)a | |
| Crude Fat | 36.4 ± 3.17 |
| Ash | 4.4 ± 0.3 |
| Mineral (mg/100 gm)a | |
| Calcium | 168 ± 10.9 |
| Phosphorus | 73.02 ± 0 |
| Fatty acids (%)a | |
| Palmitic acid (16:0) | 15.05 ± 0.65 |
| Oleic acid (18:1ω9) | 33.46 ± 1.02 |
| Stearic acid (18:0) | 3.46 ± 0.13 |
| Linoleic acid (18:2ω6) | 46.05 ± 1.3 |
| Linolenic acid(18:3ω3) | 1.98 ± 0.04 |
| ƩUSFA | 81.49 ± 2.36 |
| ƩSFA | 18.51 ± 0.78 |
| ƩUSFA/ƩSFA ratio | 4.04 |
Abbreviations: ƩUSFA, Total unsaturated fatty acids; ƩSFA, Total saturated fatty acids.
aMean of five determinations + SD.
3.2. Effect Alcoholic Extract of Nigella sativa on Kidney and Liver Lipid Peroxidelevelsor MDA Concentration
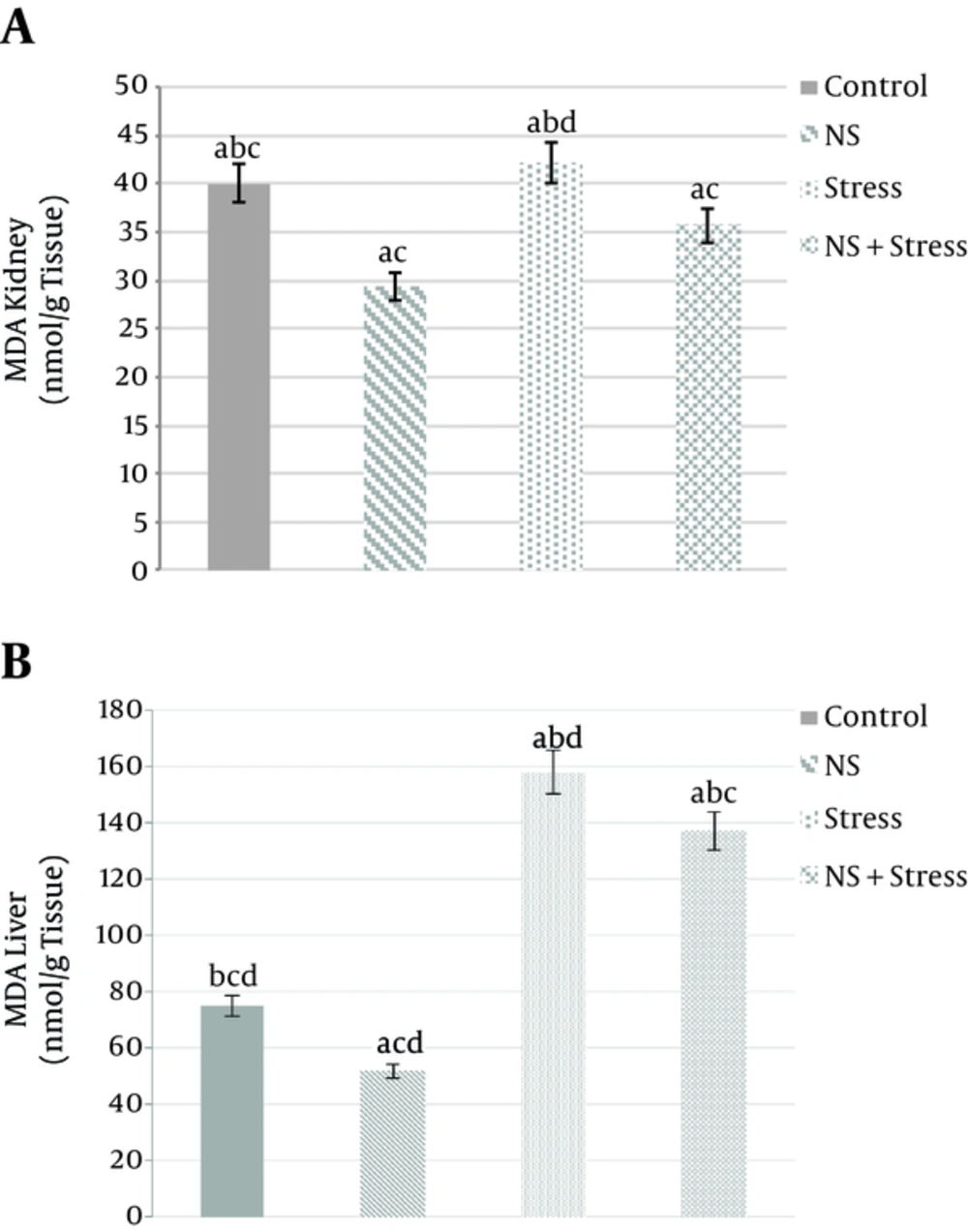
Survey data show that the kidney MDA concentration of “Nigella sativa” groups compared to the “control” groups there is a significant decrease (22.36 ± 1.06 and 40.06 ± 1.2 nmol/g of kidney, P < 0/05, respectively). In addition, the groups of “Nigella sativa + stress” (35.7±1.1nmol/g of kidney) and “Nigella sativa” (22.36 ± 1.06 nmol/g of kidney) compared to “stress” (42.17 ± 1.43 nmol/g of kidney) groups had a significant difference (P < 0/05) (Figure 1A). In addition, the liver MDA concentration in “stress” (158.17 ± 6.1 nmol/g of liver) increased almost 2 times (P < 0.05) vs “control” (75.06 ± 2.6 nmol/g of liver). Whereas, “Nigella sativa” (51.96 ± 2.1 nmol/g of liver) treatment was also found to induce a MDA concentration liver was decreased significantly when compared to the “control” groups (P < 0.05; Figure 1B). In addition, MDA concentration levels “Nigella sativa + stress” (137.33 ± 4.8 nmol/g of liver) group when compared to the control group had significant differences (P < 0/05) (Figure 1B).
Effects of alcoholic extract of Nigella sativa (NS) and stress on kidney (A) and liver (B) tissues MDA concentration. Alcoholic extract of Nigella sativa was administered in dose of 400 mg/kg/day for 3 weeks via oral. Values are expressed as mean ± SD. of 7 animals in each group. P < 0.05. A, significantly different from control; B, significantly different from nigella sativa; C, significantly different from stress; D, significantly different from “Nigella sativa + stress”. P < 0.05.
3.3. Effect of Alcoholic Extract of Nigella sativa on Kidney and Liver Tissue Ferric Reducing / Antioxidant Power (FRAP)
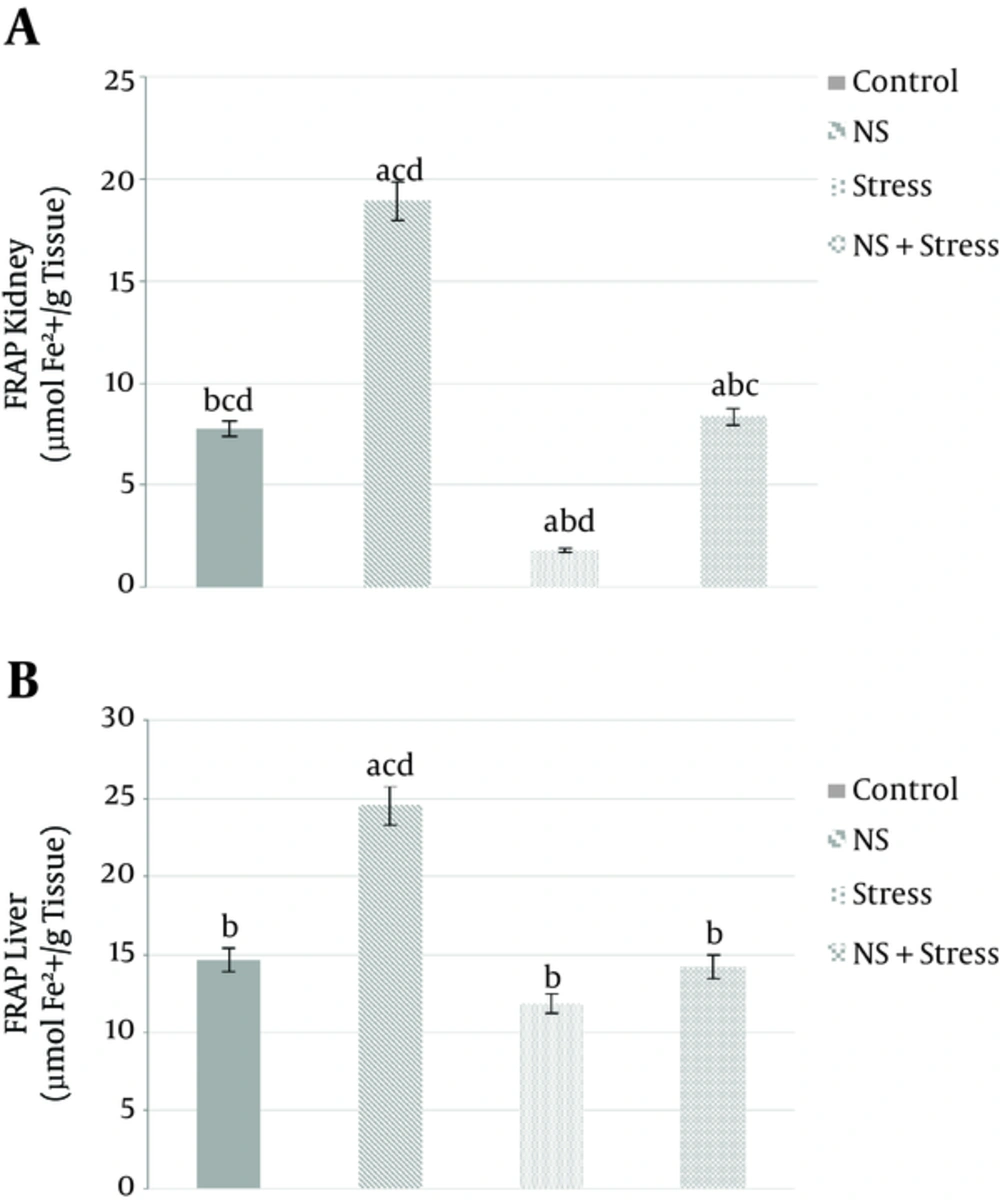
Figure 2A shows the significant differences in the amount of kidney FRAP in between all groups: “control”, “Nigella sativa”, “stress”, and “Nigella sativa + stress” were amounting (7.78 ± 1.9, 18.93 ± 3.2, 1.8 ± 0.03, 8.36 ± 1.9 µmolFe2+/g tissue, respectively. P < 0.05). Antioxidant capacity (FRAP) also significantly increased in “Nigella sativa” (24.55 ± 4.1 µmolFe2+/g tissue. P < 0.05) with respect to the control group (14.65 µmolFe2+/g tissue. P < 0.05) in liver tissue as shown in Figure 2B. Though, liver antioxidant capacity (FRAP) of “Nigella sativa + stress” group has increased compared to the “Stress” group. However, they were not significantly different between these (14.21 ± 3.3, 11.85 ± 2.8 µmolFe2+/g tissue, respectively. P < 0.05; Figure 2B).
Effects of alcoholic extract of Nigella sativa (NS) and stress on kidney (A) and liver (B) tissues Ferric Reducing / Antioxidant Power (FRAP). Alcoholic extract of Nigella sativa was administered in dose of 400 mg/kg/day for 3 weeks via oral. Values are expressed as mean ± SD. of 7 animals in each group. A, significantly different from control; B, significantly different from Nigella sativa; C, significantly different from stress; D, significantly different from “Nigella sativa + stress”. P < 0.05.
3.4. Effect of Alcoholic Extract of Nigella sativa on Kindey and Liver Catalase Activitys
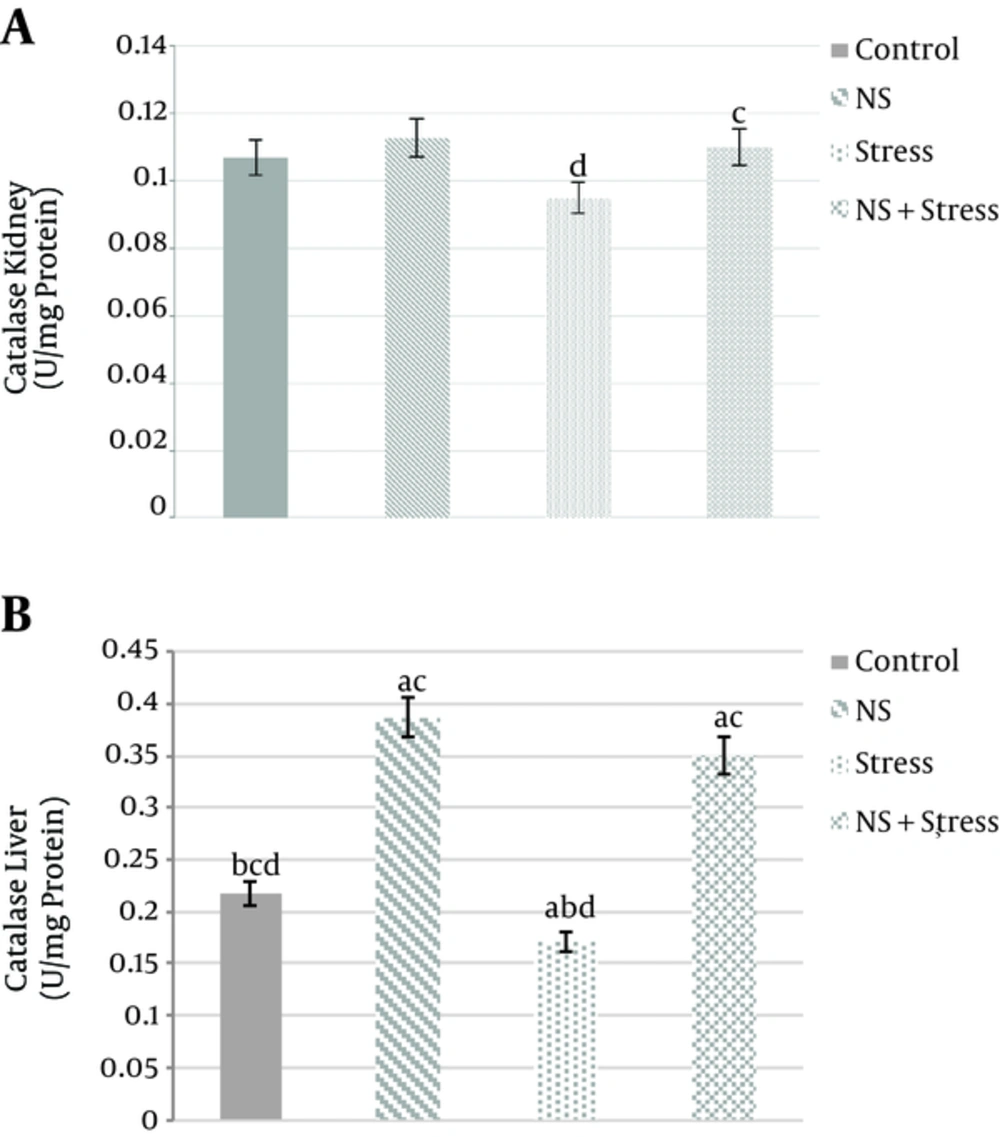
The kidney activities of CAT increased significantly in the group of “Nigella sativa + stress” (0.11 ± 0.003 U/mg protein) compared to “stress” (0.095 ± 0.002 U/mg protein) group (P < 0/05; Figure 3A). Moreover, liver of the catalase activity was significantly increased in “Nigella sativa” (0.387 ± 0.025 U/mg protein) than “control” (0.217 ± 0.018 U/mg protein) group and “Nigella sativa + stress” (0.35±0.023 U/mg protein) compared to “stress” (0.171 ± 0.014 U/mg protein) group, respectively (P < 0/05; Figure 3B).
Effects of alcoholic extract of Nigella sativa (NS) and stress on kidney (A) and liver (B) tissues Catalase (CAT) activity. Alcoholic extract of Nigella sativa was administered in dose of 400 mg/kg/day for 3 weeks via oral. Values are expressed as mean ± SD. of 7 animals in each group. A, significantly different from control; B, significantly different from Nigella sativa; C, significantly different from stress; D, significantly different from “Nigella sativa + stress” P < 0.05.
4. Discussion
The results show that crude fat (39.43%) and ash (4.4%) are in line with the ones reported by Cheikh-Rouhou et al. (2007) (3). Comparing the mineral contents of the seeds with the one reported in the literature illustrates that the differences are in calcium and phosphor amounts. It may also have resulted from geographical and climatic factor differences where Nigella seeds had been raised (3). Moreover, fatty acid compositions of the oil extracted from the seeds are presented in Table 1; this table indicates that the fatty acids contain linoleic acid, Oleic acid, Palmitic acid, Myristic acid, Linolenic acid, as reported by Cheikh-Rouhou et al., (2007) (3). The results of the nutrient contents analysis indicates that Nigella seeds are considered as a valuable source of oil and many minerals (3). Furthermore, MDA and Calcium/Phosphorus ratios in patients with rheumatoid arthritis were negatively correlated (19). The observed increase in (Ca2+) hints that secondary changes in cellular calcium homeostasis at least partially mediate the detrimental effects of free radicals (20).
Based on previous studies, compared to the liver organs, the kidney of antioxidant enzyme activities is lower (21). The results of the current research indicated that administering Nigella sativa decreases a lot of MDA concentration and increases FRAP in the liver and kidney. Moreover, it alleviates the deleterious effects of stress on kidney and liver tissues and also creates an increase in the catalase enzyme activity in the liver. Therefore, catalase activity slightly increased in kidney tissue. Studies in the literature also indicated that TQ (active ingredient Nigella sativa), at doses of 20, 40, and 80 mg / kg and Nigella sativa significantly decreased the concentration of MDA (22). In other studies, TQ increased FRAP and created a slight decrease in catalase activity (6). However, in a series studies on Nigella sativa and TQ with dose of 16 mg / kg, the MDA concentration increased slightly (23). The TQ and its metabolite (DHT) indicated as antioxidants assume an important role in precluding lipid peroxidation in different tissues (24).
Studies have shown that fish oil is rich in ω-3polyunsaturated fatty acids (PUFA), which is partially resulted from an inhibition of lipogenesis and stimulation of fatty acid oxidation in the liver. Interestingly, like other PUFAs, fish oil can be easily peroxidized to create hydroperoxides and would increase oxidative stress. Recent studies have shown that, in contrast to ω-6 PUFAs, ω-3 PUFAs inhibit the generation of free radical (25). For example, it is documented that following a long-term (6 mo) diet, rich in fish oil, increases the expression of antioxidant genes (glutathione-Stransferases, uncoupling protein-2, and Mn-SOD) in mouse liver and up regulates the expression of lipid-catabolism genes (25). Moreover, ω-3 PUFAs (docosahexaenoic acid, eicosapentaenoic acid, and α-linolenic acid) prevent iNOS expression and inducible NO synthesis by cytokine-activated macrophages (26). Thus, the beneficial effect of ω-3 PUFA on cardiovascular function is produced through 2 mechanisms: 1) reducing plasma triacylglycerol concentration and 2) preventing free radical production (27). In addition, Nigella sativa of combinations have synergistic effects. Nigella sativa precludes oxidation by combination with iron. With many diseases including cirrhosis or liver damage, which cause infection and chemical substances produced free radicals, antioxidant activity of Nigella sativa can fall very fruitful (28). In another study, it was shown that Nigella sativa reduced formation of peroxides by using iron thiocyanate methods and with the help of linoleic acid by decreasing free radicals. Moreover, this result was obtained with rapeseed oils (29). Many studies have reported the anti-oxidative effects of oil and Nigella sativa water extracts. TQ, compounds carvacrol, tetra terpineol, and the Nigella sativa have antioxidative effects (5). Therefore, Nigella sativa has antioxidant properties to help prevent free radical from having any detrimental effects on the tissues.
4.1. Conclusions
In conclusion, our results indicates that the extract of Nigella sativa has the necessary chemical characteristics to increase antioxidant capacity. Moreover, it reduces lipid peroxidation (Oxidative stress) as well as the harmful effects of stress on the body’s tissues.