1. Background
Concerns regarding health and environment, especially the safe disposal of industrial and hazardous waste, have been the topic of current interest in developing countries (1-3). Non-biodegradability of heavy metals and other metal elements in the environment can lead to be addressed for protecting the ecosystem (4-6). Industrial and hazardous waste like oily acidic sludge and oily sludge from refineries and re- refineries facilities are the main sources of pollution in some industrial zones in Iran. The final products of some facilities and industries are engine, lubricant, flux and hydraulic oil, and wastes like oily acidic sludge and filter cake (7). These wastes include a high concentration of volatile organic carbon (VOC), total petroleum hydrocarbon (TPH) and other materials that were enhanced to the oil during the initial processes (8, 9). Intentional and/or accidental discharge of these hazardous wastes into the environment, including soil and water resources, have caused some negative effects on the environment as well as human health due to different pollutants such as TPH, heavy metals and other elements in the air, and leakage of the contaminants in groundwater and soil (10). Oily sludge resulted from Re-refinery Industries contain hazardous materials that are recognized as an immunotoxicant and carcinogen agents. The acidity of the oily sludge was used in old wax refining methods in the refinery. Composting has been considered as a cost-effective and environmentally friendly method for biodegrading some wastes in the past few years (7). Aerated in-vessel composting is a technology that can easily check environmental parameters such as temperature, pH, oxygen required, nutrients, moisture content, oxidation-reduction potential, and mixing ratio. In-vessel systems need high initial investments, however, they require less space and provide better control than windrow systems; the final product by this method is more valuable (11). Composting technology has been widely applied for treating and biodegrading many types of waste such as PAHs, chlorophenols compounds, explosive materials, and polychlorobiphenyls (PCBs) (12). Simplicity, low cost, odor control, and low time reaction required for pollutant degradation are the most important advantage of this process (12). Co-composting of sludge oil and other materials and wastes is the most important characteristic of In-vessel composting.
2. Objectives
This study aimed to use in-vessel composting (effects of various environmental) factors for reducing TPH from OAS from the re-refineries industries.
3. Methods
3.1. Sampling
The present work was the experimental study that was conducted on the batch system. OAS samples were taken from refinery industry at Eshtehard industrial town (south west of Alborz province), according to the sampling protocol (13). In addition, the immature compost was provided from the Tehran composting plant (May to September, 2017 (spring and summer)). The composite composting samples were collected from first week of the composting time and various depths and then, additional materials like glass were segregated from the main mixed samples. Next, the samples were crushed to reach a size of two cm and mixed with dry manure (5% w/w) to provide required microorganisms (14).
3.2. Pilot Features
In-vessel composting reactors were plastic containers (500 mL) that were equipped to an oxygen blower, thermometer, and water supply. Diaphragm pumps (Model ACO 5505, Hailea, Guangdong, China) and distribution system was used to provide the same amount of air for each container; the amount of effluent air to each container was 20 mL/min.
3.3. Design of Experiments
3.3.1. OAS Ratio to Immature Compost
First, various ratios of OAS to compost (1:5, 1:8, 1:10, 1:15, 1:20, 1:30, 1:40, 1:50, 1:75, and 1:100) were provided and two reactors (with OAS to compost ratio was 1:0) were used for controlling the process. In addition, HgCl2 (2%) was added as biocide to one reactor, while the other reactor had no additive. The effect of aeration and volatilization process to reduce the TPH was determined in these reactors through the aeration of samples. The adaptation time to prevent the shock related to toxic materials was considered 20 days. After the passing of adaptation time, OAS was gradually injected to the reactors one time in each five days. HgCl2 was used to avoid of biological activities in the reactor.
3.3.2. Adjustment of Nutrients and Moisture Content
Nitrogen (N), phosphorus (P), and carbon (C) were formulated as 1:5:100 using NH4Cl (99.9%, Sigma-Aldrich, St. Louis, MO) as a source of N and KH2PO4 (99.5%, Sigma-Aldrich) as a source of P. The moisture content in all reactors was kept 45% to 65% duration of the composting process.
3.3.3. Analytical Methods
Before the sampling, the reactor contents were completely blended and the samples were collected in different depths. The samples were prepared with addition of 4 mL of n-hexane (99.9%, Merck, Darmstadt, Germany) 1 g of dry sample. Then, to achieve the uniform samples, the papered mixed was agitated for one minute at 300 rpm and finally the deposited particles were maintained at a refrigerator at 4°C until the analysis. GC-FID (Model CP-3800, Varian, Belrose, Australia) was used to measure the TPH. In addition, mercury thermometers and laboratory pH meter (Model, Portable Meter, Germany) were daily employed to determine the temperature and pH, respectively. To detecting pH samples at the laboratory, 1 g of dry sample was agitated in 5 mL of distilled water for one min and 200 rpm and then the supernatant was separated (13). For determining the element fluctuations during the composting process, 2 g of mixing sample was dried at 105°C for 24 hours. ICP device (Model ARCOS FHE12, Spectro, Kleve, Germany) and digestion method by nitric acid digestion was used to measure the elements (15).
4. Results and Discussion
4.1. Characteristics of OAS and Immature Compost as a Preliminary Analysis
OAS and the immature compost characteristics are presented in Table 1. As is evident from the table, TPH include more than 50% of the OAS by mass, and the pH in the obtained samples were extremely acidic (pH = 1.35). It shows that waste petroleum and acidic pH are the main issues related to the discharging these wastes to the environment. Other studies showed that the TPH contents of oily sludge were 5% to 86.2% by mass (often in the range of 15% to 50%) (10).
| Parameter | Unit | Acidic Sludge | Unmaturated Compost |
|---|---|---|---|
| Organic carbon (OC) | g.kg-1 | 539.3 | 240 |
| TKN | g.kg-1 | 10.0 | 4.61 |
| TP | g.kg-1 | 2.19 | 2.09 |
| pH | - | 1.41 | 7.31 |
| Moisture content | % | 10.6 | 46.0 |
| TPH | g.kg-1 | 520 | N.D |
| C:N ratio | - | 54.0 | 52.0 |
Table 2 shows the changes of the element concentrations in the OAS. It clears that the concentration of Zn, Cu, Fe, Al, and B in the OAS was very high and exceeded from 1000 mg.kg-1. In addition, Mo, Pb, and Mn concentrations were fairly high (between 100 - 1000 mg.kg-1). The concentration of Ni, Cr, Sn, and Li were between 47 to 69 mg.kg-1, and the concentration of other elements was less than 10 mg.kg-1. The high concentration of elements may be related to the oil interaction in the engine as well as the main source of oil. High concentrations of the elements in the oily sludge was reported in previous studies (10, 13).
| Element | Unit | Concentration |
|---|---|---|
| Zn | g.kg-1 | 5.22 |
| Cu | g.kg-1 | 4.42 |
| Fe | g.kg-1 | 5.53 |
| Al | g.kg-1 | 2.73 |
| B | g.kg-1 | 1.36 |
| Mn | g.kg-1 | 0.39 |
| Mo | g.kg-1 | 0.29 |
| Pb | g.kg-1 | 0.19 |
| Cr | mg.kg-1 | 69.1 |
| Sn | mg.kg-1 | 58.6 |
| Ni | mg.kg-1 | 56.0 |
| Li | mg.kg-1 | 47.9 |
| V | mg.kg-1 | 8.28 |
| As | mg.kg-1 | 4.96 |
| Cd | mg.kg-1 | 4.44 |
| Co | mg.kg-1 | 3.78 |
| Hg | mg.kg-1 | 3.49 |
4.2. TPH Removal in Composting Reactors
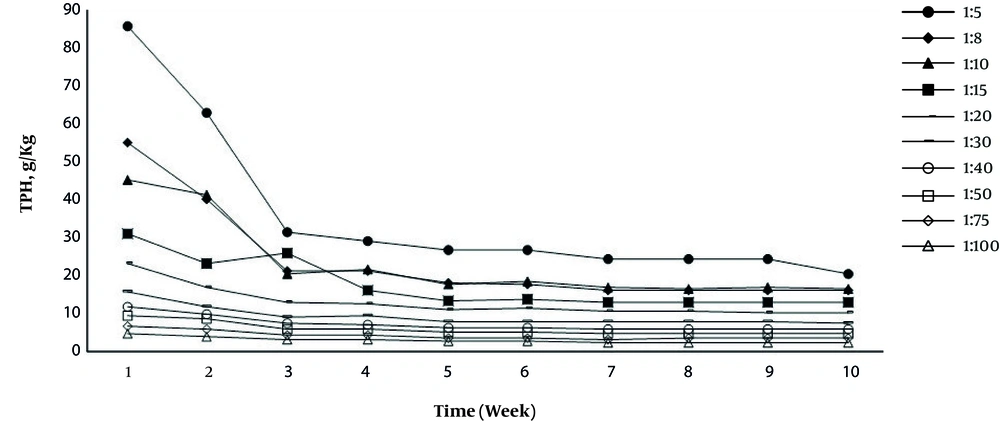
TPH removal efficiency during the composting period are shown in Table 3. According to the results, the highest and lowest concentration of TPH at adaptation time after 10 weeks was obtained in the sludge to compost ratio of 1:5 and 1:100, respectively. It means that 83.9 and 2.80 g of the TPH were removed. Decreasing the removal efficiency of the biodegradation process was more than 48% in all reactors; however, the highest removal efficiency was obtained in the sludge to compost ratio of 1:5 and 1:8 that was 71.6% 70.6%. The maximum removal efficiency compared to the initial amount that actually occurred within the first three weeks indicates that the high amount the hydrocarbons (mainly saturated fractions) was decomposed. Figure 1 illustrates the changes of TPH removal efficiency during the composting process. The minimum TPH removal efficiency was obtained at a mixing ratio of 1:100 (48.5%).
| Time (Week) | Removal Efficiency (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1:5 Ratio | 1:8 Ratio | 1:10 Ratio | 1:15 Ratio | 1:20 Ratio | 1:30 Ratio | 1:40 Ratio | 1:50 Ratio | 1:75 Ratio | 1:100 Ratio | |
| 0 | 18.1 | 13.0 | 8.50 | 6.80 | 5.90 | 10.9 | 9.00 | 11.3 | 7.00 | 10.3 |
| 2 | 27.0 | 27.0 | 9.10 | 24.6 | 26.7 | 23.4 | 16.5 | 6.90 | 6.90 | 16.9 |
| 3 | 64.4 | 61.1 | 55.1 | 47.2 | 43.5 | 40.9 | 38.3 | 35.8 | 33.0 | 36.4 |
| 4 | 66.0 | 61.3 | 52.7 | 48.5 | 45.6 | 40.5 | 40.2 | 36.3 | 36.7 | 35.8 |
| 5 | 68.8 | 67.2 | 61.4 | 56.5 | 52.3 | 49.6 | 47.7 | 44.3 | 43.4 | 44.0 |
| 6 | 68.9 | 67.6 | 59.5 | 55.0 | 51.0 | 49.2 | 46.5 | 45.2 | 43.3 | 42.8 |
| 7 | 71.8 | 70.8 | 62.8 | 58.7 | 55.0 | 50.9 | 50.7 | 49.4 | 49.4 | 48.5 |
| 8 | 71.6 | 70.9 | 63.3 | 58.3 | 55.0 | 50.3 | 51.2 | 50.1 | 48.3 | 48.7 |
| 9 | 71.5 | 70.9 | 62.9 | 58.2 | 55.5 | 50.9 | 51.6 | 50.5 | 49.0 | 49.2 |
| 10 | 71.6 | 70.9 | 63.3 | 58.3 | 55.5 | 51.1 | 51.5 | 50.1 | 49.2 | 48.5 |
The TPH removal efficiency was higher than 60% at mixing ratios of less than 1:10 and 48.5% to 58.3% for mixing ratios of more than 1:10. Thus, the effects of mixing ratios on the removal of organic compounds and TPH was significant. These results are similar to previous studies (8, 12, 13). The results also revealed that the mixing ratios of more than 1:10 need further compost to mitigate the contamination. In addition, the removal efficiency of TPH at the mixing ratios of more than 1:10 increase insignificantly in comparison with less than 1:10. Therefore, increasing organic amendment will reduce efficiency of TPH reduction (16).
4.3. Monitoring Parameters (pH and Temperature)
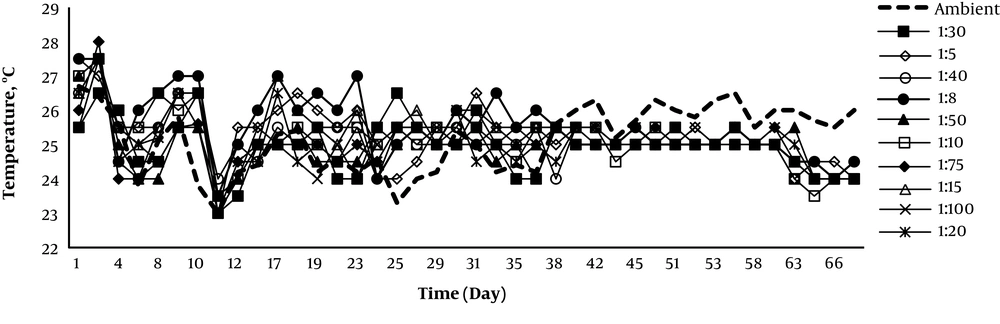
Figures 2 and 3 represent the temperature and pH monitoring for the 10 weeks of experiments. The reactor temperature was above the ambient in the first six weeks. Temperature variations indicate the microorganism activity during the composting process. The correlation between the temperature and biological reaction rate of composting process was very strong (17). Reducing the temperature after 6 weeks presents a diminution of the biodegradable material. Since small reactors were used for this study, the temperatures set were higher. The maximum and minimum temperatures of composting reactors and ambient air were 28.0°C, 23.0°C, 26.5°C, and 23.0°C (mesophilic temperature), respectively. According to these results (Figures 1 and 2), when the temperature of reactor was reduced less than ambient temperature, the removal efficiency of TPH was very slow. Therefore, the reactor temperature has a considerable effect on the biodegradation of TPH during the composting process. These results were confirmed by other studies (12, 13, 18).
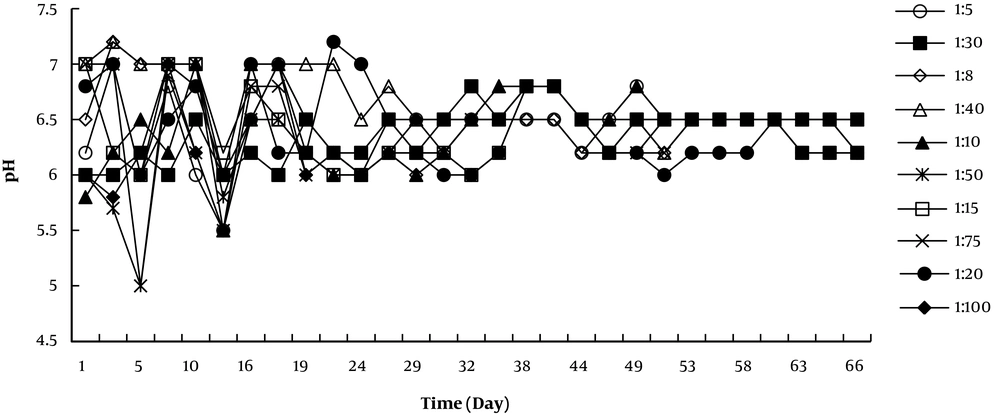
The pH plays an important role in the microbial activity. The pH value in this study, at the initial stages, varied between 6.70 and 7.20 and then dropped (about 5.50) after two weeks. It was due to the decomposition of organic matter and the production of acidic agents such as organic acids and carbon dioxide during the microbial activities in the reactors (17, 19). In addition, after four weeks, the pH value was in the range of 6.50 to 7.00 during the nitrogen compound mineralization (Figure 3). The significant relationship between temperature, time, and pH and degradation of organic materials was observed.
4.4. Volatilization and Abiotic Degradation
According to the results, the total volatilization loss with and without HgCl2 during the composting process after 10 weeks were 1.22% and 2.00%, respectively. Thus, the results of this study showed that the roles of abiotic degradation and volatilization were minor (less than 5.00%). The temperature employed in this survey was lower than the temperature which might have significantly impacted on the TPH removal by volatilization (8, 20). Therefore, biodegradation and biological process during the composting process is the main cause of TPH degradation. According to the other studies, microbial activity was the main mechanism of TPH removal in biodegradation of petroleum waste in the composting technology (8, 21).
4.5. Elements Analysis
The results of the analyses of elements for four times after the adjustment periods are shown in Tables 4 and 5. After mixing the OAS with immature compost in 10 ratios, the initial concentrations of the elements decreased so that for concentrations below 10 mg.kg-1 in mixing ratios greater than 1:8, the values changed to µg.kg-1 (Table 5). ANOVA test results showed that the changes in time were not effective on the average concentrations of the elements (P = 0.99). Thus, the concentration of metals in the composting process would not be changed. In previous studies, the concentrations of heavy metals had increased with the passage of time (11, 22). The highest and lowest percentages of increased concentrations of metals in the mixing ratios are as follows: Al: 1:30 (36.3%) to 1:10 (3.63%), Fe: 1:50 (20.1%) to 1:8 (0.84%), Zn: 1:20 (23.0%) to 1:75 (0.68%), Cu: 1:75 (13.0%) to 1:15 (0.88%), B: 1:75 (38.3%) to 1:8 (0.66%), Mn: 1:5 (16.8%) to 1:20 (0.85%), Pb: 1:5 (9.11%) to 1:40 (1.48%), Mo: 1:8 (6.06%) to 1:5 (1.34%), Cr: 1:50 (13.1%) to 1:20 (0.72%), Sn: 1:40 (6.54%) to 1:8 (3.16%), Ni: 1:50 (5.80%) to 1:10 (0.93%), Li: 1:75 (5.88%) to 1:5 (1.58%), As: 1:50 (66.1%) to 1:10 (1.75%), Cd: 1:8 (25.0%) to 1:50 (1.94%), V: 1:100 (12.9%) to 1:50 (0.57%), Co: 1:75 (10.8%) to 1:100 (3.60%), and Hg: 1:5 (14.2%) to 1:50(0.63%), respectively. The statistical analysis showed that the relationship between concentration of metals (the highest and lowest percentages) were significant (P value < 0.05). Reduction of organic matter was listed as the main reason for the increase in concentrations of metals during composting in previous studies, (11, 22). According to Figure 1 and Table 1, the highest TPH removal efficiency occurred in the ratios of 1:5 (71.6%), 1:8 (70.6%), and 1:10 (63.3%), respectively. The minimum concentrations of elements for the three mentioned ratios were 1:10, 1:8, and 1:5, respectively. The minimum and maximum concentration reduction in the ratio of 1:8 to 1:5, 1.11 (Cd) to 2.04 (Co), the ratio of 1:10 to 1:5, 1.50 (As) to 4.25 (B), and the ratio of 1:10 to 1:8 was equivalent to 0.89 (Co) to 3.38 (B) times, respectively. The statistical analysis showed that the relationship between concentration of metals (the minimum and maximum) were significant (P value < 0.05). Thus, the ratio of 1:10 is the best option for removal of TPH and reduction of elements’ concentrations. Previous studies showed that concentrations of zinc were higher than those of copper, the selenium was even lower (23).
| Time (Week) | Concentration of Elements (mg.kg-1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1:5 Ratio | 1:8 Ratio | 1:10 Ratio | 1:15 Ratio | 1:20 Ratio | 1:30 Ratio | 1:40 Ratio | 1:50 Ratio | 1:75 Ratio | 1:100 Ratio | |
| Al | ||||||||||
| 2 | 1149 | 655 | 468 | 227 | 200 | 165 | 141 | 104 | 88.0 | 53.0 |
| 4 | 1053 | 783 | 457 | 204 | 203 | 202 | 163 | 112 | 93.0 | 60.0 |
| 6 | 1096 | 702 | 425 | 211 | 182 | 248 | 169 | 94 | 101 | 59.0 |
| 8 | 1071 | 882 | 485 | 292 | 221 | 225 | 166 | 119 | 105 | 56.0 |
| Fe | ||||||||||
| 2 | 1792 | 1097 | 811 | 658 | 451 | 352 | 252 | 152 | 134 | 85.1 |
| 4 | 1836 | 963 | 836 | 583 | 473 | 334 | 247 | 146 | 130 | 88.2 |
| 6 | 1955 | 1147 | 838 | 655 | 474 | 345 | 217 | 167 | 119 | 82.1 |
| 8 | 1899 | 1106 | 888 | 607 | 470 | 338 | 247 | 182 | 130 | 89.0 |
| Zn | ||||||||||
| 2 | 1737 | 1146 | 817 | 573 | 375 | 291 | 195 | 148 | 111 | 65.6 |
| 4 | 1771 | 1102 | 857 | 510 | 437 | 266 | 199 | 135 | 98.9 | 60.7 |
| 6 | 1513 | 1170 | 789 | 555 | 418 | 301 | 194 | 132 | 108 | 56.8 |
| 8 | 1685 | 1172 | 880 | 593 | 462 | 296 | 198 | 141 | 112 | 62.6 |
| Cu | ||||||||||
| 2 | 1559 | 990 | 642 | 558 | 364 | 267 | 184 | 156 | 121 | 68.0 |
| 4 | 1401 | 742 | 660 | 515 | 402 | 297 | 177 | 153 | 104 | 59.2 |
| 6 | 1400 | 924 | 605 | 523 | 384 | 311 | 195 | 146 | 141 | 75.9 |
| 8 | 1544 | 943 | 675 | 553 | 395 | 296 | 194 | 161 | 137 | 68.8 |
| B | ||||||||||
| 2 | 563 | 453 | 181 | 183 | 123 | 70.7 | 70.6 | 34.8 | 27.2 | 21.4 |
| 4 | 663 | 418 | 216 | 184 | 94.5 | 95.2 | 79.4 | 49.0 | 28.4 | 25.3 |
| 6 | 507 | 403 | 119 | 161 | 67.2 | 82.8 | 73.8 | 22.6 | 32.8 | 27.4 |
| 8 | 600 | 456 | 245 | 191 | 128.5 | 80.6 | 85.5 | 41.1 | 37.6 | 27.9 |
| Mn | ||||||||||
| 2 | 122 | 72.5 | 51.5 | 41.8 | 27.1 | 21.8 | 14.4 | 10.1 | 8.50 | 6.31 |
| 4 | 132 | 72.0 | 52.3 | 38.7 | 22.8 | 22.8 | 13.1 | 9.66 | 9.00 | 5.63 |
| 6 | 120 | 73.1 | 53.8 | 43.9 | 25.2 | 22.4 | 14.9 | 9.79 | 8.72 | 5.70 |
| 8 | 143 | 73.1 | 53.2 | 43.2 | 27.3 | 22.3 | 14.1 | 10.2 | 8.59 | 6.43 |
| Pb | ||||||||||
| 2 | 61.3 | 39.1 | 27.3 | 22.8 | 14.2 | 10.1 | 6.74 | 5.34 | 3.86 | 2.40 |
| 4 | 58.7 | 42.7 | 26.1 | 21.9 | 13.3 | 10.4 | 6.29 | 5.51 | 3.55 | 2.36 |
| 6 | 60.7 | 36.4 | 27.0 | 19.7 | 15.1 | 8.15 | 6.22 | 5.70 | 4.08 | 2.39 |
| 8 | 66.9 | 39.7 | 28.3 | 23.6 | 13.8 | 10.5 | 6.84 | 5.52 | 3.67 | 2.22 |
| Mo | ||||||||||
| 2 | 97.9 | 61.8 | 44.2 | 34.4 | 23.3 | 16.1 | 10.6 | 7.87 | 5.46 | 2.59 |
| 4 | 82.8 | 69.0 | 41.4 | 29.7 | 22.1 | 14.7 | 10.9 | 8.58 | 5.30 | 2.82 |
| 6 | 96.4 | 73.2 | 40.6 | 32.2 | 22.3 | 14.0 | 10.2 | 7.72 | 6.16 | 2.76 |
| 8 | 99.2 | 65.6 | 42.2 | 34.9 | 22.1 | 16.8 | 10 | 7.64 | 5.73 | 2.47 |
| Cr | ||||||||||
| 2 | 28.7 | 17.5 | 13.7 | 10.1 | 6.88 | 5.95 | 3.93 | 2.36 | 1.64 | 1.05 |
| 4 | 24.4 | 20.7 | 11.3 | 10.0 | 6.51 | 5.83 | 3.92 | 2.63 | 1.40 | 1.02 |
| 6 | 29.6 | 19.6 | 12.1 | 10.3 | 6.41 | 5.42 | 3.98 | 2.48 | 1.65 | 1.02 |
| 8 | 28.2 | 18.8 | 13.5 | 10.5 | 6.93 | 6.05 | 3.99 | 2.67 | 1.62 | 1.11 |
| Sn | ||||||||||
| 2 | 17.8 | 11.7 | 7.34 | 6.26 | 4.30 | 3.43 | 2.14 | 1.66 | 1.27 | 0.71 |
| 4 | 16.7 | 12.0 | 7.29 | 6.65 | 4.39 | 3.28 | 2.37 | 1.57 | 1.21 | 0.66 |
| 6 | 18.7 | 12.1 | 7.43 | 6.74 | 4.10 | 3.54 | 2.42 | 1.72 | 1.20 | 0.68 |
| 8 | 18.5 | 12.1 | 7.67 | 6.54 | 4.51 | 3.12 | 2.28 | 1.73 | 1.32 | 0.70 |
| Ni | ||||||||||
| 2 | 16.8 | 11.3 | 7.48 | 6.07 | 4.09 | 3.08 | 1.99 | 1.55 | 1.16 | 0.68 |
| 4 | 17.4 | 11.3 | 6.89 | 5.80 | 3.99 | 2.94 | 1.88 | 1.44 | 1.08 | 0.65 |
| 6 | 17.73 | 11.2 | 6.61 | 5.92 | 4.07 | 2.90 | 1.80 | 1.52 | 1.19 | 0.62 |
| 8 | 16.31 | 11.3 | 7.55 | 6.19 | 4.18 | 3.05 | 1.90 | 1.64 | 1.19 | 0.66 |
| Li | ||||||||||
| 2 | 13.85 | 8.96 | 5.86 | 5.10 | 3.42 | 2.67 | 1.71 | 1.19 | 1.02 | 0.75 |
| 4 | 13.63 | 9.94 | 5.94 | 5.21 | 3.94 | 2.66 | 1.56 | 1.11 | 0.93 | 0.69 |
| 6 | 13.06 | 9.22 | 6.00 | 4.94 | 3.78 | 3.05 | 1.79 | 1.15 | 0.98 | 0.78 |
| 8 | 14.07 | 9.41 | 6.19 | 5.03 | 3.57 | 2.73 | 1.81 | 1.17 | 1.08 | 0.69 |
| Time (Week) | Concentration of Elements (µg.kg-1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1:5 Ratio | 1:8 Ratio | 1:10 Ratio | 1:15 Ratio | 1:20 Ratio | 1:30 Ratio | 1:40 Ratio | 1:50 Ratio | 1:75 Ratio | 1:100 Ratio | |
| As | ||||||||||
| 2 | 2371 | 2076 | 1556 | 1300 | 735 | 585 | 488 | 196 | 191 | 98.3 |
| 4 | 2516 | 2214 | 1047 | 730 | 597 | 771 | 338 | 247 | 198 | 97.3 |
| 6 | 2684 | 2157 | 1790 | 1009 | 725 | 864 | 277 | 185 | 184 | 110 |
| 8 | 2425 | 2132 | 1583 | 1250 | 857 | 539 | 645 | 326 | 178 | 110 |
| Cd | ||||||||||
| 2 | 1194 | 752 | 675 | 576 | 490 | 336 | 283 | 200 | 156 | 83.7 |
| 4 | 1665 | 980 | 819 | 698 | 541 | 336 | 276 | 215 | 150 | 79.6 |
| 6 | 1169 | 1053 | 753 | 697 | 474 | 398 | 240 | 195 | 146 | 83.7 |
| 8 | 1257 | 941 | 738 | 619 | 583 | 332 | 258 | 204 | 155 | 86.6 |
| V | ||||||||||
| 2 | 2760 | 1765 | 1178 | 951 | 579 | 487 | 305 | 236 | 168 | 97.5 |
| 4 | 2735 | 1842 | 1155 | 944 | 568 | 482 | 295 | 243 | 171 | 104 |
| 6 | 2617 | 1904 | 1231 | 959 | 580 | 487 | 292 | 231 | 175 | 114 |
| 8 | 2784 | 1771 | 1170 | 990 | 589 | 457 | 321 | 237 | 180 | 110 |
| Co | ||||||||||
| 2 | 1362 | 957 | 721 | 605 | 482 | 350 | 234 | 171 | 90.8 | 61.9 |
| 4 | 1565 | 924 | 649 | 564 | 413 | 277 | 221 | 181 | 89.9 | 60.1 |
| 6 | 1431 | 703 | 789 | 344 | 378 | 330 | 228 | 194 | 94.0 | 63.4 |
| 8 | 1449 | 909 | 672 | 513 | 472 | 324 | 226 | 169 | 101 | 64.1 |
| Hg | ||||||||||
| 2 | 1116 | 667 | 470 | 393 | 284 | 161 | 125 | 91.9 | 65.3 | 46.0 |
| 4 | 1229 | 636 | 384 | 391 | 300 | 164 | 131 | 90.8 | 76.8 | 47.5 |
| 6 | 1160 | 618 | 440 | 379 | 267 | 148 | 130 | 92.9 | 70.3 | 46.5 |
| 8 | 1275 | 700 | 475 | 403 | 286 | 172 | 127 | 92.5 | 68.2 | 46.1 |
5. Conclusion
Based on the results of this study, combination of OAS and composting materials can significantly reduce TPH. In addition, reduction of the TPH (more than 63.0%) and element concentrations was significant at mixing ratio (sludge to compost) of 10:1. Nevertheless, the residual TPH and elements concentrations are higher than existing environmental standards, however, addition of immature compost as an amendment in in-vessel composting could be considered as an effective method for minimization of TPH.