1. Background
Organophosphate (OP) poisoning is a major global health issue caused by exposure to OP compounds, which are widely used as pesticides. Organophosphates are among the most common causes of accidental, occupational, and suicidal intoxications due to their widespread use, easy accessibility, and simplicity in synthesis (1). Despite the role of pesticides in enhancing agricultural productivity, concerns are increasing about their adverse effects on human health (2). Organophosphate poisoning is more prevalent in developing countries where highly hazardous pesticides (HHPs) are more readily available. Significant incidences of intentional or unintentional OP poisoning occur in the agricultural regions of developing countries in South Asia and Southeast Asia, including China, India, Sri Lanka, Iran, and the Maldives (2, 3). Annually, the global death toll from OP poisoning is estimated to range from 250 000 to 350 000 (4). In rural areas, poisoning with organophosphorus compounds typically occurs deliberately with suicidal intent (5). One study reported that 51.7% of OP poisonings were deliberate, while 21.7% were accidental (4). Previous studies have indicated that intentional self-poisoning is associated with significantly higher mortality than unintentional poisoning (6). Given the high prevalence of intentional poisoning with organophosphorus compounds, particularly in developing countries, and the high mortality associated with it, implementing necessary therapeutic interventions for these patients is crucial.
Organophosphates inhibit the enzyme acetylcholinesterase (AChE) by phosphorylating the serine hydroxyl group on the enzyme. Acetylcholinesterase is responsible for hydrolyzing the neurotransmitter acetylcholine (ACh) at cholinergic synapses. Therefore, AChE inhibition leads to the accumulation of ACh at the cholinergic synapse, resulting in prolonged stimulation of nicotinic and muscarinic ACh receptors, which manifests as OP toxidrome (1, 7). In addition to supportive care, the standard treatment includes three main therapeutic approaches: Atropine to block muscarinic receptors, oxime-type reactivators (e.g., pralidoxime, obidoxime) to dephosphorylate the inhibited AChE and benzodiazepines for seizure management.
In Iran, pralidoxime chloride (2-PAM) is the oxime of choice and is commonly used as an antidote for OP poisoning. It works by reactivating AChE, thus reducing the accumulation of ACh (8). For adults, the initial recommended dose of pralidoxime chloride typically ranges from 1 to 2 grams, followed by a continuous infusion at a rate of 250 to 500 mg per hour. However, dosing guidelines can vary based on the specific product used and the severity of the poisoning. Close monitoring of the patient's clinical response is crucial to determine the need for additional doses or adjustments in the infusion rate. In pediatric patients, the dose of pralidoxime is generally calculated based on body weight, with an initial dose typically ranging from 20 to 50 mg/kg, followed by a continuous infusion at a rate of 5 to 10 mg/kg per hour (9-11).
There is a divergence of opinion regarding the effectiveness of pralidoxime in treating OP poisoning; some studies have reported that its administration leads to a decrease in mortality among patients poisoned with organophosphorus agents (12-15), while others indicate no effect (16, 17). Several previous studies examining the effectiveness of various dosages of pralidoxime are outlined in Table 1 (11, 14, 16, 18-25). The appropriate dose of pralidoxime for a specific individual depends on several factors, including the severity of poisoning, age, weight, and individual response (26). Additionally, some studies recommend high-dose pralidoxime (12), while others have reported that high doses may be associated with a higher mortality rate and recommend low-dose pralidoxime (27). With the expansion of information technology in many research fields, including healthcare, real-world studies such as non-interventional and observational studies have become significant data sources for clinical research (28). Compared to traditional models, machine learning approaches offer immense benefits in handling real-world evidence. Unlike traditional models, which cannot handle complex, interacting, high-dimensional variables, machine learning models are more accurate and provide greater generalizations (29). The use of machine learning techniques based on real-world research has become popular recently, with examples including models for predicting tacrolimus blood concentration in patients with autoimmune diseases, the prediction of vancomycin dose through XGBoost, and the prediction of warfarin maintenance dose through LightGBM (30-32).
| Authors | Study | Country, Year | Number of Patients | Sex | Age (y) | Pralidoxime Dosage | Outcome |
|---|---|---|---|---|---|---|---|
| Pawar et al. (14) | RCT | India, 2006 | 200, poisoning by anticholinesterase pesticide | Male; control group: 52; study group: 57 | Control group: 29 (22 - 35); Study group: 28 (22 - 33) | Control group: 2 g loading dose over 30 min, then a bolus dose of 1 g/4 h for 48 h; study group: 2 g loading dose over 30 min, then a constant infusion of 1 g/h for 48 h. | Patients in the study group required less atropine, a short duration of ventilatory support, and less frequency of intubation. |
| Syed et al. (16) | RCT | India, 2015 | 100 patients with OP poisoning | Sex ratio (male:female); control group: 1:1.56; study group: 1:1.62 | Control group: 28.2 ± 9.9; study group: 29.1 ± 10.9 | Control group: Received I.V saline; study group: (30 mg/kg loading dose over 30 min followed by 8 mg/kg/h continuous infusion for a maximum of 7 days | There was no statistically significant difference between the two groups in terms of mortality, hemodynamic parameters, atropine requirements, duration of ventilation, and ICU stay |
| Eddleston et al. (11) | RCT | Sri Lanka, 2009 | 235 patients with OP poisoning | Male; control group: 92 (80.7); study group: 96 (79.3) | Control group: 29.5 (23 - 42); study group: 31 (22 - 48) | Control group: Normal saline infusion; study group: 2 g loading dose over 20 min, then a constant infusion of 0.5 g/h until 7 days | Mortality was non-significantly higher in patients receiving pralidoxime, and the need for intubation was similar in both groups |
| Thunga and Pandey (18) | Cross-sectional, nonrandomized observational study | India, 2013 | 256 OPs poisoned patients | Sex ratio (male: female): 2.3:1 | Majority of patients were in the age group of 21 - 30 years | Control group: Normal saline infusion; study groups: Intermittent (1 g/q8h), continuous infusion (500 mg/h), continuous infusion (1 g/h) | The incidence of intermediate syndrome, number of ventilation days, total atropine requirement, number of hospitalization days, and mortality rate significantly reduced in continuous infusion of pralidoxime at 500 mg/hour |
| Due (19) | Comparative study | Vietnam, 2014 | Control group: 54; study group: 108 | Male; control group: 30 (55.6); study group: 64 (59.3) | Control group: 25.5 ± 10; study group: 29.5 ± 14.2 | Control group: Received a mean total dose of 7.2 ± 4.1 g; study group: Received a mean total dose of 20.0 ± 12.7 g | In the study group, patients received significantly lower doses of atropine and required a shorter duration of hospital stay, and the mortality rate was lower than the control group |
| Mahesh et al. (20) | Randomized open-labeled prospective study | India, 2013 | Control group: 45; study group: 37 | Male:female, control group: 35:10; study group: 21:16 | Control group: 30.1 ± 7.3; study group: 31.3 ± 8.9 | Control group: 2 g bolus followed by 1g/6 h; study group: 2 g bolus followed by 8 mg/kg/h infusion | Duration of mechanical ventilation, mean dosage of atropine administered, and incidence of intermediate syndrome were significantly lower in the study group than in the control group |
| Chaudhary et al. (21) | Case-control study | India, 2013 | Control group: 35; study group: 35 | Male:female, control group: 25:10; study group: 24:11 | Control group: 24.80 ± 8.3; study group: 25.17 ± 9.2 | Control group: -; study group: 30 mg/kg body weight in 200 mL of normal saline over 30 min followed by 2.0 g in 200 mL of normal saline over 30 min, at 6 h intervals for initial 72 h | There was no statistically significant disparity observed between the two groups in relation to the total amount of atropine administered, necessity for intubation, average length of hospitalization, and mortality rate. |
| Cherian et al. (22) | RCT | India, 2005 | Control group: 11; study group: 10 | NA | NA | Control group: Normal saline infusion; Study group: PAM infusion of 12 g/day for 3 days in severe cases and 4 g/day for 3 days in moderate cases | There was no difference between the treatment and placebo groups in relation to the necessity for intubation, average length of hospitalization, and mortality rate. |
| Cherian et al. (23) | RCT | India, 1997 | Control group: 55; Study group: 55 | Male, control group: 34 (62); study group: 41 (75) | Control group: 26.5 ± 10.3; study group: 28 ± 10.1 | Control group: Normal saline infusion for 3 days; study group: Infusion of 12 g over 3 days | The requirement of ventilator support (37 vs. 22), the incidence of an intermediate syndrome (36 vs. 19), and the mortality rate (16 vs. 3) were higher in the study group than in the control group |
| Banerjee et al. (24) | Open-label, parallel-group clinical study | India, 2011 | Control group: 30; study group: 30 | Male, control group: 11 (37); study group: 14 (47) | Control group: 34.3 ± 8.8; study group: 34.6 ± 9.8 | Control group: -; study group: a dose of 0.5 - 1 g/6 h | The mortality rate, requirement of ventilator, and duration of hospital stay in the two groups failed to show any statistically significant difference. |
| Banerjee et al. (25) | Open-label, parallel-group, randomized clinical trial | India, 2014 | Control group: 60; study group: 60 | Male, control group: 26 (43); study group: 23 (38) | Control group: 34.3 ± 8.8; study group: 34.6 ± 9.8 | Control group: -; study group: A dose of 1 g every 6 hours for a period of 5 days | Pralidoxime therapy did not offer any appreciable benefit over atropine alone in terms of reducing mortality and ventilator requirement. Patients in the study group experienced longer duration of hospital stay |
Characteristics of Previous Studies Investigating the Effectiveness of Pralidoxime in Patients with Organophosphates Poisoning a
2. Objectives
In this study, we aimed to predict an optimal pralidoxime therapeutic dose based on real-world evidence, including initial symptoms, clinical presentation, vital signs, and laboratory parameters at presentation, using machine learning methods to enhance its efficacy in patients poisoned by OPs.
3. Methods
3.1. Study Population
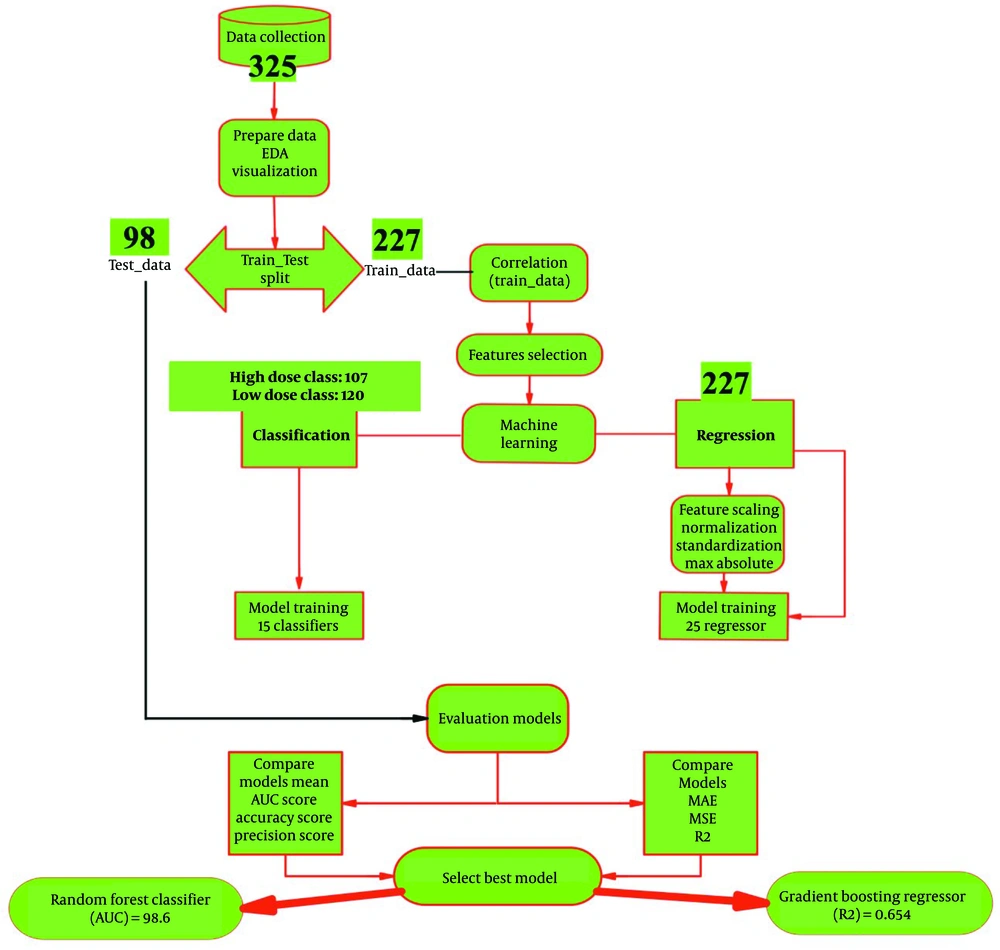
The roadmap of the proposed system for predicting pralidoxime dose in patients with OP poisoning is illustrated in Figure 1. This retrospective study was conducted at Loghman Hakim Hospital, Tehran, Iran's primary referral center for poisoned patients, from March 2016 to April 2021. The study included patients aged 14 years and older who had been poisoned with OPs and met specific criteria. These criteria included documented OP exposure and a plasma cholinesterase (PChE) activity of less than 4300 U/L upon admission, indicative of acute OP poisoning (33). A total of 325 patients were included, particularly those who received commonly recommended pralidoxime dose regimens. These regimens typically involved an initial intravenous infusion of 1 - 2 g (10 - 20 mg/mL) over 15 - 30 minutes, followed by a continuous infusion of 500 mg/h until 24 hours after the patient was weaned from atropine. The study aimed to predict the outcome of dried pulmonary secretions and adequate oxygenation following pralidoxime administration. Exclusion criteria included non-receipt of pralidoxime, multidrug toxicity, and the presence of severe chronic comorbidities. Ethical approval for the study was obtained from the ethics committee (IR.SBMU.RETECH.REC.1401.257), and informed consent was obtained from all participants.
3.2. Collection and Processing of Data
Data for this study were obtained from electronic medical records. Two researchers reviewed the medical records of the patients, and a questionnaire was used to extract clinical information from the electronic databases of Loghman Hakim Hospital. The questionnaire covered various patient demographics, medical history, symptoms, and laboratory test results, as well as hospital-related factors such as the need for intubation and duration of hospitalization. The dataset underwent several preparation processes, including the removal of rows with missing values greater than 70%, application of min-max scalar and standard scalar, data validation, under-sampling, and splitting of the dataset.
3.3. Feature Selection
Feature selection in machine learning involves identifying the most relevant attributes or parameters to improve model performance. In this research, the filter approach using Pearson’s correlation coefficient was employed to select features by assessing the correlation between each variable and the predictive outcome. The aim was to identify the key predictors of the total pralidoxime dose necessary for maintaining sufficient oxygenation. Only variables demonstrating a strong correlation with the predictive outcome, such as dried pulmonary secretions and adequate oxygenation, were retained through the feature selection process. Variables with zero correlation coefficients were excluded from the feature subset, while those with high coefficients were included in the selected variables subset.
3.4. Statistical Analysis
Based on the outcomes of the Kolmogorov-Smirnov and Shapiro-Wilk tests, it was determined that all continuous variables exhibited non-normal distributions. Consequently, these variables were represented by their median values and interquartile ranges and were analyzed using the Kruskal-Wallis and Mann-Whitney U tests. Categorical variables were depicted in terms of absolute frequencies (n) and were analyzed using the chi-square test.
3.5. Model Development and Performance Evaluation
The prediction model for pralidoxime dose in patients poisoned by OPs was assessed using two methods: (1) Regression, with pralidoxime dose considered as a continuous variable; and (2) classification, with pralidoxime dose divided into two classes, low dose, and high dose. In the low dose class, patients received total doses of less than 14 000 g, and in the high dose class, patients received total doses of 14 000 g or more. For the first method, 25 machine learning models were used, and for the second method, 15 machine learning models were trained. Models with the best performance are listed in Table 2 (the top ten models for the regression method) and Table 3 (the top 14 models for the classification method). A ten-fold cross-validation technique was employed to train and test the machine learning algorithms using both complete and selected feature datasets. This method involves dividing the dataset into ten sections and performing the holdout method ten times. The final dataset was randomly split into training (70%) and testing (30%) sets. Model creation, hyper-parameter tuning, and model performance evaluation were conducted using these datasets. This approach prevents random data bias and ensures an equal distribution of data between the training and test sets. It is important to note that the testing dataset was not used during the training process. Hyperparameters were tuned using the training dataset through the cross-validation method. Python software was used to develop a model utilizing classification models. The performance of the classification algorithms was assessed by testing them on the testing dataset after training. Five commonly used efficiency testing metrics, including sensitivity, specificity, accuracy, F1-score, and AUC, were utilized to evaluate the effectiveness of classification algorithms in predicting the pralidoxime dose in OP-poisoned patients. These performance measures were then used to compare the performance of each trained classifier with that of other machine-learning systems. The efficiency evaluation metrics for the classifiers are as follows:
Four performance measures were employed in the regression method to evaluate the performance of each algorithm: Mean absolute error (MAE), mean squared error (MSE), root mean squared error (RMSE), and R-squared (R2). The calculations for these metrics are as follows:
In the process of regression analysis, we employed the standardization technique to scale the variables. Standardization involves centering the values around the mean and adjusting them to have a unit standard deviation. This is achieved by dividing the variable by its standard deviation after subtracting the mean, as indicated by the following equation:
| Index | Model | MAE | MSE | RMSE | R2 | RMSLE | MAPE |
|---|---|---|---|---|---|---|---|
| Gbr | Gradient boosting regressor | 9208.1856 | 265695067.5245 | 15210.5268 | 0.6540 | 0.7210 | 0.8991 |
| light gum | Light gradient boosting machine | 11082.3208 | 411516903.5829 | 18637.1318 | 0.5818 | 0.8653 | 1.2540 |
| Et | Extra trees regressor | 9964.7046 | 390583726.4613 | 17809.5835 | 0.4884 | 0.7287 | 0.9194 |
| Ada | Adaboost regressor | 14937.2844 | 428589688.6916 | 19027.8917 | 0.4837 | 1.1143 | 2.2290 |
| xgboost | Extreme gradient boosting | 9210.9117 | 342642130.7921 | 17211.7812 | 0.4199 | 0.7087 | 0.9305 |
| Rf | Random forest regressor | 11670.8163 | 433264351.1262 | 19619.4416 | 0.4145 | 0.7812 | 1.0514 |
| Huber | Huber regressor | 16389.3648 | 1018552916.3411 | 27720.5679 | 0.1742 | 1.1506 | 1.2774 |
| Par | Passive aggressive regressor | 16079.8772 | 1110867086.3911 | 29187.1724 | 0.0923 | 1.1319 | 1.0646 |
| En | Elastic net | 19619.8389 | 1026946561.5622 | 29350.4610 | 0.0035 | 1.2072 | 2.2364 |
| Dt | Decision tree regressor | 10381.8142 | 832112670.2866 | 24221.9796 | 0.0017 | 0.8261 | 0.7595 |
Results of Ten-Fold Cross-Validation for Regressors Performance on Selected Predictors After Feature Scaling in Regression Method
| Index | Model | Accuracy | AUC | Recall | Precision | F1 | Kappa |
|---|---|---|---|---|---|---|---|
| rf | Random forest classifier | 0.9152 | 0.9865 | 0.9061 | 0.9308 | 0.9169 | 0.8307 |
| light gum | Light gradient boosting machine | 0.9073 | 0.9756 | 0.9159 | 0.9129 | 0.9107 | 0.8145 |
| gbc | Gradient boosting classifier | 0.9071 | 0.9584 | 0.9318 | 0.896 | 0.9125 | 0.8136 |
| ada | Ada boost classifier | 0.8897 | 0.9218 | 0.897 | 0.8968 | 0.8945 | 0.7786 |
| et | Extra trees classifier | 0.8798 | 0.9619 | 0.872 | 0.9013 | 0.883 | 0.7599 |
| xgboost | Extreme gradient boosting | 0.8763 | 0.9548 | 0.8826 | 0.881 | 0.879 | 0.7528 |
| lda | Linear discriminant analysis | 0.8275 | 0.8515 | 0.8379 | 0.8314 | 0.8328 | 0.6544 |
| ridge | Ridge classifier | 0.7966 | 0.0 | 0.8212 | 0.7942 | 0.8044 | 0.5924 |
| dt | Decision tree classifier | 0.7832 | 0.7839 | 0.7879 | 0.7967 | 0.7829 | 0.5672 |
| lr | Logistic regression | 0.748 | 0.8254 | 0.7871 | 0.751 | 0.7651 | 0.4947 |
| qda | Quadratic discriminant analysis | 0.7069 | 0.8036 | 0.6477 | 0.826 | 0.6635 | 0.4168 |
| knn | K neighbors classifier | 0.6947 | 0.7909 | 0.7015 | 0.7225 | 0.7043 | 0.3898 |
| nb | Naive bayes | 0.6856 | 0.7843 | 0.7682 | 0.6856 | 0.7195 | 0.363 |
| Svm | SVM - linear kernel | 0.5796 | 0.0 | 0.5705 | 0.6766 | 0.5375 | 0.1577 |
Results of Ten-Fold Cross-Validation for Classifiers Performance on Selected Predictors in Classification Method
4. Results
4.1. Patients
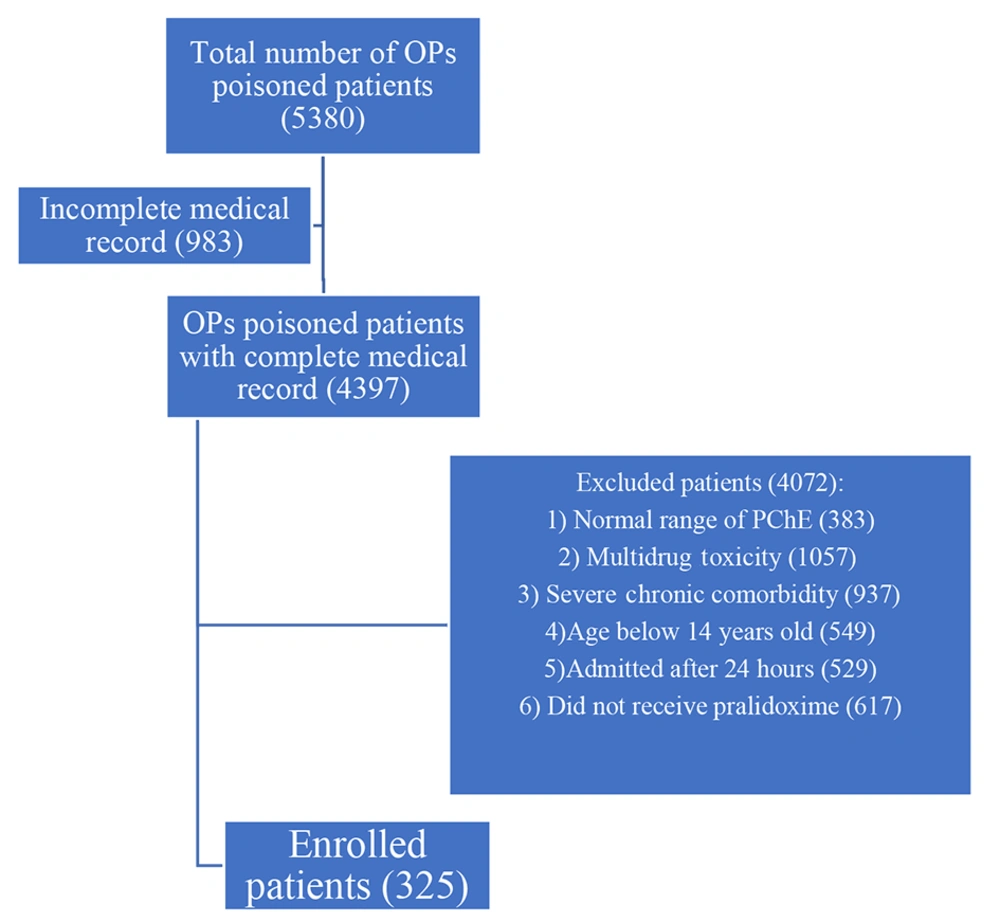
After a comprehensive review of electronic medical records, a total of 5,380 patients diagnosed with OP poisoning were identified within the database, forming the primary cohort. Among them, 983 patients had incomplete medical records, and 4,072 patients were excluded based on predetermined exclusion criteria. Specifically, 383 patients had a normal range of PChE, 1,057 patients had multidrug toxicity, 937 patients had chronic comorbidity, 529 patients were admitted more than 24 hours after poisoning, 549 patients were below 14 years of age, and 617 patients did not receive pralidoxime. Ultimately, 325 patients were enrolled in the study. Figure 2 depicts the patient selection process. The descriptive characteristics of the study sample are presented in Table 4.
| Variables | Pralidoxime Dose | Total (n = 325) | P-Value | |
|---|---|---|---|---|
| Low Dose (n = 172) | High Dose (n = 153) | |||
| Sex | 0.06 | |||
| Male | 96 | 69 | 165 | |
| Female | 76 | 84 | 160 | |
| Age | 33 (25) | 31 (22) | - | 0.092 |
| Time to hospitalization | 4 (10) | 4 (7) | - | 0.332 |
| Sialorrhea | 0.000 | |||
| No | 160 | 135 | 295 | |
| Yes | 12 | 18 | 30 | |
| Bradycardia | 0.370 | |||
| No | 169 | 152 | 321 | |
| Yes | 3 | 1 | 4 | |
| Rales | 0.000 | |||
| No | 153 | 111 | 264 | |
| Yes | 171 | 153 | 324 | |
| Bronchospasm | 0.467 | |||
| No | 116 | 97 | 213 | |
| Yes | 56 | 56 | 112 | |
| Intubation | 0.763 | |||
| No | 121 | 105 | 226 | |
| Yes | 51 | 48 | 99 | |
| Incontinence | 0.138 | |||
| No | 166 | 142 | 308 | |
| Yes | 6 | 11 | 17 | |
| Fasciculation | 0.000 | |||
| No | 160 | 122 | 282 | |
| Yes | 12 | 31 | 43 | |
| Muscular weakness | 0.000 | |||
| No | 141 | 47 | 188 | |
| Yes | 31 | 106 | 137 | |
| Paralysis | 0.000 | |||
| No | 172 | 140 | 312 | |
| Yes | 0 | 13 | 13 | |
| Tachycardia | 0.738 | |||
| No | 88 | 75 | 163 | |
| Yes | 84 | 78 | 162 | |
| Confusion | 0.270 | |||
| No | 98 | 96 | 194 | |
| Yes | 74 | 57 | 131 | |
| Lethargy | 0.180 | |||
| No | 170 | 153 | 323 | |
| Yes | 2 | 0 | 2 | |
| Coma | 0.380 | |||
| No | 127 | 106 | 233 | |
| Yes | 45 | 47 | 92 | |
| Agitation | 0.017 | |||
| No | 172 | 148 | 320 | |
| Yes | 0 | 5 | 5 | |
| Seizures | 0.621 | |||
| No | 167 | 147 | 314 | |
| Yes | 5 | 6 | 11 | |
| ICU admission | 0.284 | |||
| No | 95 | 75 | 170 | |
| Yes | 77 | 78 | 155 | |
| The amount of toxin consumed | 90 (120) | 150 (150) | 100 | 0.00 |
| GCS | 15 (7) | 15 (7) | 12.5 | 0.589 |
| Cholinesterase level first | 305 (971) | 317 (1114) | 316.5 | 0.787 |
| Cholinesterase level last | 458 (835) | 715 (1898) | 583.50 | 0.172 |
| PH-VBG | 7.35 (0.11) | 7.34 (0.13) | 7.345 | 0.291 |
| PCO2-VBG | 38.4 (14.4) | 41.5 (11.7) | 40.3 | 0.001 |
| HCO3-VBG | 19.6 (4.7) | 20.4 (5.2) | 20.4 | 0.346 |
| Cr | 1.2 (0.3) | 1.2 (0.3) | 1.2 | 0.005 |
| BUN | 28 (18.5) | 33 (15) | 31 | 0.002 |
| AST | 26 (16.3) | 25 (12) | 25 | 0.595 |
| ALT | 19 (13) | 17 (9) | 18 | 0.017 |
| ALP | 204 (79) | 178 (79) | 192 | 0.002 |
| Bulos of atropine | 0.5 (1) | 1 (1) | 0.5 | 0.593 |
The Study Sample's Descriptive Characteristics a
4.2. Feature Selection
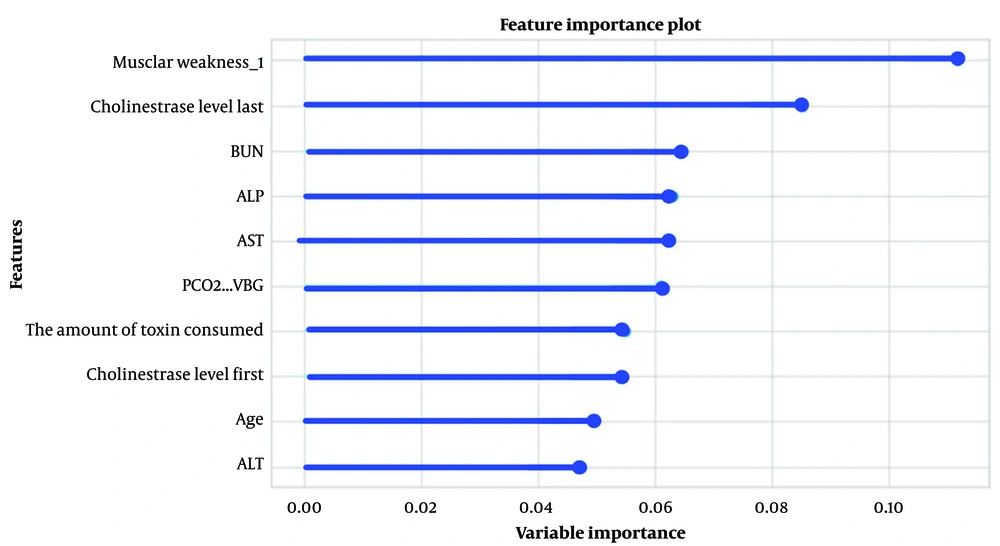
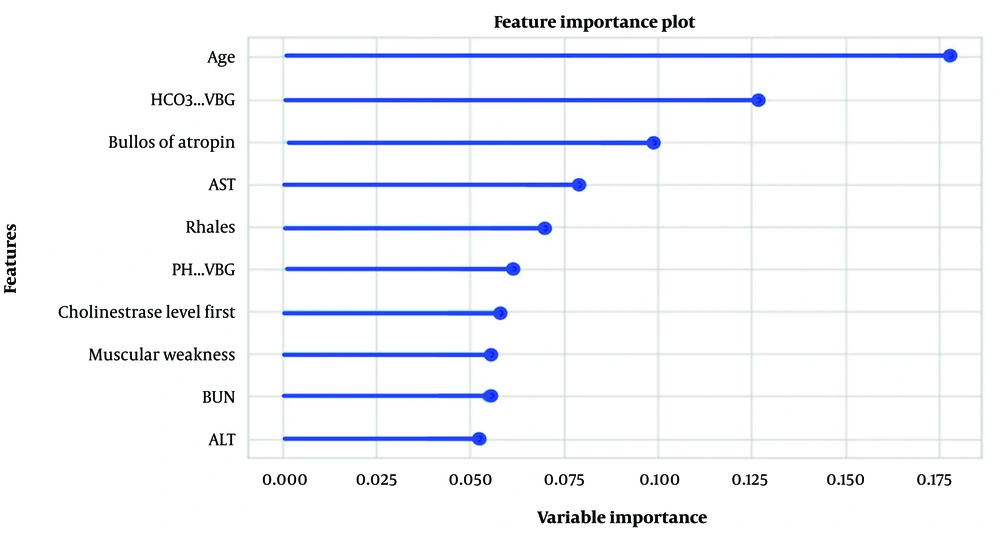
Significant and relevant features for predicting the pralidoxime dose in OP-poisoned patients were selected using Pearson’s correlation coefficient (filter approach). Figure 3 shows the top ten variables selected for the classification method, where muscular weakness, PChE activity, and blood urea nitrogen (BUN) scored the highest for predicting pralidoxime dose. In the regression method, after scaling the variables using the standardization technique, the top ten features in predicting the dose of pralidoxime from most to least important were age, HCO3-VBG, bolus of atropine, aspartate aminotransferase (AST), pulmonary rale, PH-VBG, PChE activity, muscular weakness, BUN, and alanine transaminase (ALT) (Figure 4).
4.3. Performance of Prediction Models
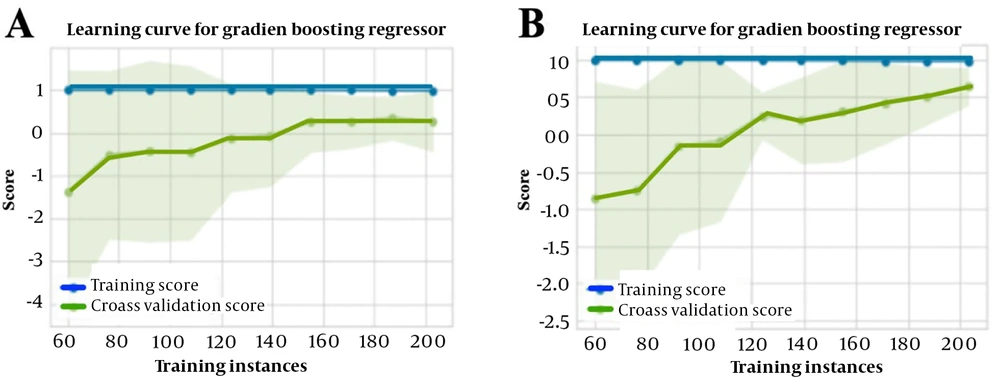
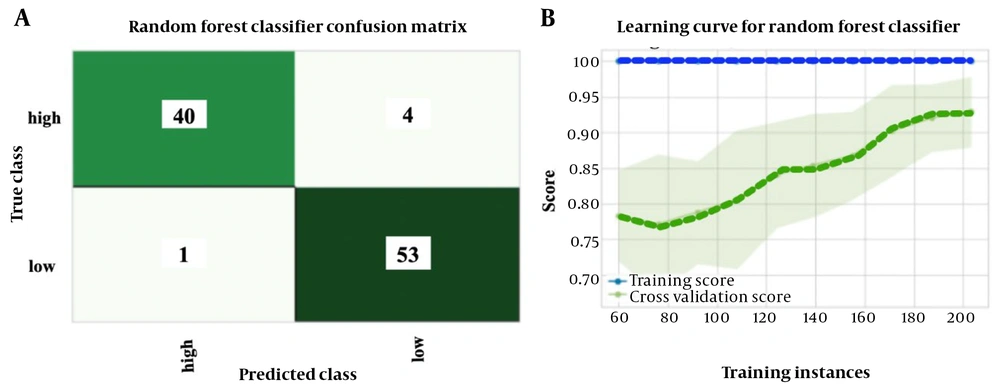
Performance metrics were calculated to compare the effectiveness of prediction models in classification and regression methods. Table 2 presents the results of the top ten prediction algorithms for the regression method using selected standard features to predict the pralidoxime dose in patients with OP poisoning. The gradient-boosting regressor emerged as the most accurate and responsive model, exhibiting the highest R2 value. This prediction model had an MAE of 9208.19, an MSE of 265695067.52, an RMSE of 15210.52, and an R2 of 65.4. Figure 5 depicts the learning curves of the regression method before (A) and after (B) feature scaling, demonstrating improved convergence of training and cross-validation scores after scaling. Table 3 summarizes the results of the top 14 prediction algorithms for the classification method using selected features to predict the pralidoxime dose in OP-poisoned patients. Based on ten-fold cross-validation findings, the random forest classifier showed the highest accuracy and sensitivity, as well as the highest AUC value among the classifiers tested. This model achieved a mean accuracy of 91.5%, a recall or sensitivity of 90.6%, a precision of 93.1%, an F1-score of 91.7%, Kappa statistics of 83.1%, and an AUC of 98.6. Figure 6 presents the confusion matrix (A) and learning curve (B) for this model, indicating a good fit as the training and cross-validation curves converge at a high score.
5. Discussion
Immediate antidotal treatment in patients with severe OP poisoning is crucial. The first line of antidotal therapy for OP poisoning includes atropine and pralidoxime (34). In antidote therapy, prescribing the correct dose of the antidote is vital, although the appropriate dose of pralidoxime in patients with OP poisoning remains controversial, with various dosage schedules being employed (20, 35). Several studies have investigated the ideal dose of pralidoxime in OP-poisoned patients, but the results have been inconclusive. For instance, one study reported that a pralidoxime dose of 20.0 ± 12.7 g was more effective than a dose of 7.2 ± 4.1 g, leading to a reduction in the duration of hospitalization and mortality rate (19). Another study found that a pralidoxime dose of 2 g followed by an 8 mg/kg/h infusion was more effective than a dose of 2 g followed by 1 g every 6 hours (20). We hypothesize that an individualized pralidoxime dose, determined based on clinical presentations and laboratory parameters, might be helpful in these cases.
Machine learning techniques enable healthcare providers and researchers to leverage large amounts of patient data to make precise and individualized dose predictions. By tailoring treatment plans to the unique characteristics of each patient, machine learning can contribute to improved treatment outcomes, reduced adverse effects, and enhanced patient safety. Machine learning algorithms can analyze complex patterns and relationships within patient data to make accurate dose predictions (36). For instance, in one study, researchers used machine learning methods to develop a predictive model for determining the appropriate dosage of lapatinib in patients with metastatic HER2(+) breast cancer, utilizing real-world data and applying both machine learning and deep learning techniques (29). Another study reported an optimal dosing algorithm for vancomycin in patients with MRSA and other gram-positive bacterial infections, developed using machine-learning methods (31). Asiimwe et al. assessed the performance of 21 machine-learning algorithms in predicting stable warfarin doses in sub-Saharan Black-African patients (37).
In this study, we applied a machine learning technique for the first time to predict pralidoxime doses in patients poisoned by OPs based on demographic profiles, clinical symptoms, and laboratory parameters. We employed two different methods for this purpose. The first method, classification, grouped patients into two categories based on the pralidoxime dose: Either low dose (< 14000 g) or high dose (≥ 14000 g). In the second method, after feature scaling using the standardization technique, the pralidoxime dose was included in the machine-learning model as a continuous variable. A total of 35 features were selected for both the classification and regression methods. The following features were among the top ten in both methods: Age, muscular weakness, ALT, AST, BUN, and initial PChE level. Apart from these shared features, in the classification method, the last measured level of PChE (last measurement within the first 24 hours of hospitalization), ALP, PCO2-VBG, and the amount of consumed toxin was among the top ten predictors of pralidoxime. In the regression method, additional top predictors included HCO3-VBG, received a bolus of atropine, pulmonary rale, and PH-VBG.
Many of the features selected in this study overlap with those identified in our previous study, which examined predictors of OP poisoning severity (38). The AChE enzyme prevents the buildup of the neurotransmitter ACh at various muscarinic and nicotinic sites in the body by hydrolyzing it. This enzyme has both a serine site and an anionic site. The OP molecule attacks the serine site located within the active site of the enzyme. As a result, through an irreversible interaction, the serine site is phosphorylated, and a strong covalent bond is formed, leading to the inactivation of the enzyme's active site. Pralidoxime functions as a reactivator of AChE by attaching to the enzyme's anionic site, close to the previously attached OP molecule at the serine site. The pralidoxime molecule sacrifices itself by becoming phosphorylated instead of the enzyme since it has a higher affinity for phosphorylation by OP agents than the enzyme's serine site. Consequently, the OP molecule detaches from the enzyme and forms an OP-pralidoxime complex, which is then hydrolyzed. This process results in enzyme reactivation (39). The main effect of pralidoxime is the restoration of ACh esterase at nicotinic sites in the body, alleviating symptoms such as muscle weakness, fasciculations, and paralysis (40). According to our results, muscular weakness was one of the most significant features in predicting the dose of pralidoxime, selected in both the classification and regression methods. Other predictors of pralidoxime dosage are also features directly related to the severity of organophosphorus poisoning and can be used to predict the dosage of pralidoxime in these patients.
In the classification method among different machine learning algorithms, the random forest classifier demonstrated the strongest performance, with an AUC value of 98.6%. In the regression method, the gradient-boosting regressor showed the strongest performance, with an R2 value of 0.65. Both the random forest classifier and the gradient boosting regressor are decision-tree-based machine learning algorithms. Algorithms utilizing decision trees demonstrate high efficacy in predictive modeling, especially for small-to-medium structured datasets with substantial missing data (38).
The novelty of this study lies in the utilization of machine learning methodology to forecast the optimal dosage of pralidoxime for individuals affected by OP poisoning. This investigation marks the inaugural development of a machine learning-based predictive model for pralidoxime dosage, employing two distinct approaches: Classification and regression. The strength of the study lies in the use of machine learning methods, which offer numerous advantages such as enhanced accuracy, efficiency, and decision-making capabilities. Nevertheless, the study is subject to several limitations, notably the small sample size and the retrospective nature of the data, which led to a considerable amount of missing data in the dataset. Additionally, the absence of an external validation cohort and the recruitment of patients solely from a single hospital further constrain the study. Thus, to obtain confirmatory results, a more robust multicenter study with a larger sample size is imperative.
5.1. Conclusions
Machine learning-based prediction algorithms can be utilized to anticipate the appropriate dosage of pralidoxime for patients suffering from organophosphorus (OP) poisoning. Through the application of feature selection techniques, age, HCO3-VBG, received bolus of atropine, and AST were identified as the most significant predictors of pralidoxime dosage in the regression method. The gradient-boosting regressor emerged as the most effective algorithm with the highest predictive performance. In the classification method, muscular weakness, the last measured level of PChE, BUN, ALP, and AST were determined to be the most crucial predictors of pralidoxime dosage, with the random forest classifier demonstrating the best predictive performance. These algorithms have the potential to contribute to the development of a clinical decision support system for the precise individualized dosing of pralidoxime in OP poisoning cases. Nevertheless, further research with a larger sample size is imperative to substantiate these findings.