1. Background
Water pollution caused by petroleum hydrocarbons can occur through various means, such as produced water in petroleum extraction, fuel spills during the transport of petroleum products, leakage from storage tanks and pipes, and fuel spills at gas stations, as well as from cars, vehicles, and other sources. Petroleum hydrocarbons contain about 64% aliphatic hydrocarbons, 33% aromatics, 1% olefins, and 0.5% BTEX (1, 2). Water pollution caused by diesel is considered more harmful than pollution caused by crude oil (3). This is because diesel fuel undergoes physicochemical changes, and when mixed with water, it quickly disperses and can remain in the water for an extended period, also dispersing in water in the form of micro and nano droplets (4).
Research has been conducted for the removal of petroleum hydrocarbons from water using bacteria, such as the removal of petroleum hydrocarbons from water by bacteria isolated from oil-contaminated soil (5), the removal of crude oil from saline water by three species of Acinetobacter (6), Proteus vulgaris isolated from fuel-contaminated water (7), Proteus isolated from petroleum oil sludge (8), Acinetobacter and fungi (9), Alcaligenes (10), and biodegradation of petroleum hydrocarbons using Enterobacter and Acinetobacter (11). There are some studies on the removal of petroleum hydrocarbons with the combined system of plants and bacteria, such as using bacterial flocs formed around Azolla roots (12), adding bacterial culture to the floating aquatic plant system (13), by bacterial flocs developed around the dead Azolla pinnata fronds (14), by phytoremediation combined with bacterial inoculation (15), and bioremediation of alkane hydrocarbons using a bacterial consortium from soil (16).
Studies have also been conducted using Azolla for the remediation of petroleum hydrocarbons from water, such as the biodegradation of crude fuel in water by A. pinnata (17), the removal of crude oil from water by A. filiculoides (18), and pyrocatechol removal from aqueous solutions using A. filiculoides (19). Azolla is a unique plant with distinct characteristics; it is an aquatic fern that grows and reproduces on the surface of water and develops many roots below the water surface, which can provide bacteria with oxygen, nutrients, and a suitable surface for the formation of bacterial biofilm (20). Thus, Azolla is a promising candidate for phytoremediation (21). The coexistence of nitrogen-fixing bacteria (cyanobacteria) with Azolla enables the plant to absorb and fix nitrogen from the air (22).
Using a combined method of phytoremediation and bacterial degradation to remove petroleum hydrocarbons from water improves removal efficiency because plant roots secrete compounds such as carbohydrates, organic acids, and enzymes into the water, which feed the bacteria. The roots provide oxygen for aerobic bacteria and also a contact surface for bacterial biofilm formation. Additionally, bacteria can help the plant survive by degrading and reducing hydrocarbon concentrations. Bacteria also cause more dispersion of petroleum compounds in the water by secreting surfactant emulsifiers, which results in easier absorption by the plant roots (23).
2. Objectives
The aim of this study was to remove diesel fuel from water by co-culturing Azolla and bacteria using isolated bacteria from soil polluted by diesel fuel and to compare the efficiency with cultures of bacteria and Azolla alone.
3. Methods
3.1. Propagation of Azolla
Azolla filiculoides was collected from a paddy field in Sari, Iran, and cultivated under artificial light with an intensity of 10.0 k lux, at a temperature of 30°C, using Hoagland hydroponic growth medium with (1.0 M, mL per liter: 5.0 Ca(NO3)2, 5.0 KNO3, 2.0 MgSO4·7H2O, 1.0 KH2PO4, and 1.0 mL micronutrient solution).
3.2. Co-culturing of Bacteria and Azolla
Bushnell-Hass (BH) broth (gL-1: 1.0 KH2PO4, 1.0 K2HPO4, 1.0 (NH4)2SO4, 0.2 MgSO4, 0.05 FeCl3, and 0.02 CaCl2, pH = 7) enriched by 1.0 gL-1 of glucose was used for bacterial culture. Commercial diesel fuel was sterilized, and predetermined amounts of it were mixed with the culture medium and then sonicated in the presence of an emulsifier (0.01% of Tween 80) for 30 minutes to prepare 100, 500, and 1000 mgL-1 of total petroleum hydrocarbon (TPH). The selection of TPH concentrations tested in this study was based on reports from articles on determining TPH concentrations in water following oil spills from tankers, which reported TPH concentrations in water in the range of 100 to 1000 mg/L (24, 25).
The bacterial isolation was performed using BH mineral medium containing 1000 mg/L of TPH, focusing on the ability to use this compound as the main substrate of bacteria. The isolates with different appearances were purified on nutrient agar and blood agar medium. Then, isolates were selected and purified based on their apparent characteristics and growth rate on the mineral medium containing diesel fuel. Classifying and identifying the bacteria was performed based on the morphological characteristics of colonies on the medium and standard biochemical tests (26).
To investigate the hydrocarbon-decomposing ability of bacteria, each of the isolated bacterial cells was transferred to 100 mL of mineral medium containing diesel fuel and incubated at 30°C for 7 days. A mineral medium containing diesel fuel, without any bacterial inoculation and plant, was used as the positive control. The bacterial culture media that showed high growth ability after seven days were selected to evaluate the ability to remove hydrocarbons from water (27). The Bergey’s Manual, 9th Edition, was used as a presumptive characterization and bacterial identification method (28).
In the experiments involving bacteria, a bacterial inoculum of 100 mL, consisting of a suspension of each isolated bacterium in water, was added separately to the culture medium containing TPH. In the experiments involving Azolla, 3.0 grams of healthy fresh Azolla fronds were placed into the flasks containing glucose-enriched growth medium polluted by TPH in two different modes: With and without bacterial inoculum. Experiments were conducted over a period of 14 days in three different modes: Azolla cultivation, bacterial cultivation, and combined cultivation (bacteria and Azolla). The cultivation was carried out in an incubator with a light/dark cycle of 16/8 hours, day and night cycle, and a temperature of 30°C, with a light intensity of 10,000 lux.
Positive controls, without plants and bacteria, were used to determine the amount of evaporated TPH, while a negative control, without TPH, was used to confirm the growth of Azolla. In all flasks, the evaporated water was replenished by adding distilled water. Sampling was done on days 5, 10, and 14 after the start of cultivation. Samples were prepared by extraction of water with dichloromethane and analyzed by standard method (29).
3.3. Statistical Analysis
The data were analyzed using two-way analysis of variance (ANOVA) with SPSS 24 software at a significance level (P-value) of 0.05. Independent variables (factors) were: Treatment type (3.0 levels: Azolla, bacteria, Azolla + bacteria) and contact times. The normality of data was checked by the Shapiro-Wilk test, and the homogeneity of variances was checked by Levene’s test. Figure 1 shows a test flask containing Azolla in water contaminated by diesel fuel.
4. Results
4.1. Characterization and Identification of Bacteria
Isolated bacteria from diesel-contaminated soil were identified by biochemical test methods, and the results are shown in Table 1.
| Strains | Biochemical Tests | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAT | STH | GEL | TSI | LAC | SUC | MAL | CIT | VP | MR | UR | IND | MAN | H2S | XYS | GLU | LYS | NI | MOT | XI | GR | |
| A1 | + | - | - | N | - | - | - | + | - | - | - | - | - | - | - | - | - | - | + | + | + |
| A2 | + | - | - | A/A | + | + | - | + | + | - | - | - | + | - | + | + | + | + | + | - | - |
| A3 | + | - | - | K/K | - | - | - | + | - | - | - | - | + | - | + | + | - | - | - | - | - |
| A4 | + | - | + | A/A | - | - | - | + | - | + | + | - | - | + | + | + | - | + | + | - | - |
| A5 | + | - | + | K/K | - | - | - | + | - | - | - | - | - | - | - | - | - | + | + | + | - |
Abbreviations: A1, Alcaligenes faecalis; CAT, catalase; STH, starch hydrolysis; GEL, gelatin hydrolysis; TSI, triple sugar iron agar; LAC, lactose; SUC, sucrose; MAL, maltose; CIT, citrate; VP, Voges-Proskauer; MR, methyl red test; UR, urease; IND, indole; MAN, MANOSE; XYS, xylose; GLU, glucose; LYS, lysine; NI, nitrate; MOT, motility; XI, oxidase; GR, gram reaction; A2, Enterobacter aerogenes; A3, Acinetobacter baumannii; A4, Proteus vulgaris; A5, Pseudomonas aeruginosa.
4.2. Removal of Total Petroleum Hydrocarbon by Azolla
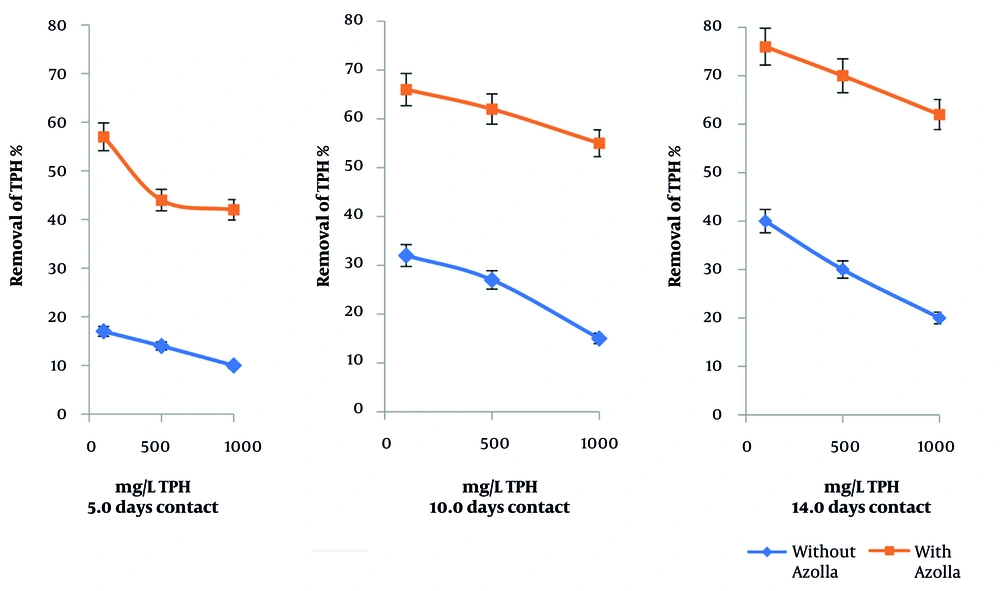
Figure 2 shows the removal of 100, 500, and 1000 mgL-1 of TPH from water using 3.0 grams of Azolla at contact times of 5.0, 10.0, and 14.0 days.
The graphs in Figure 2 illustrate that the flask containing Azolla achieved a TPH removal efficiency of 57% after 5.0 days of contact with a TPH concentration of 100 mg/L. However, when the TPH concentration was increased to 1000 mgL-1, the removal efficiency decreased to 42%. Under the same conditions, in the flask without Azolla, evaporation resulted in a reduction of TPH concentration by 28% and 18% for initial TPH concentrations of 100 mgL-1 and 1000 mgL-1, respectively. Furthermore, Figure 2 illustrates that the TPH removal efficiency increased significantly for all three TPH concentrations as the contact time was extended to 10 and 14 days.
4.3. Removal of Total Petroleum Hydrocarbon by Bacteria and Combined Azolla-Bacteria
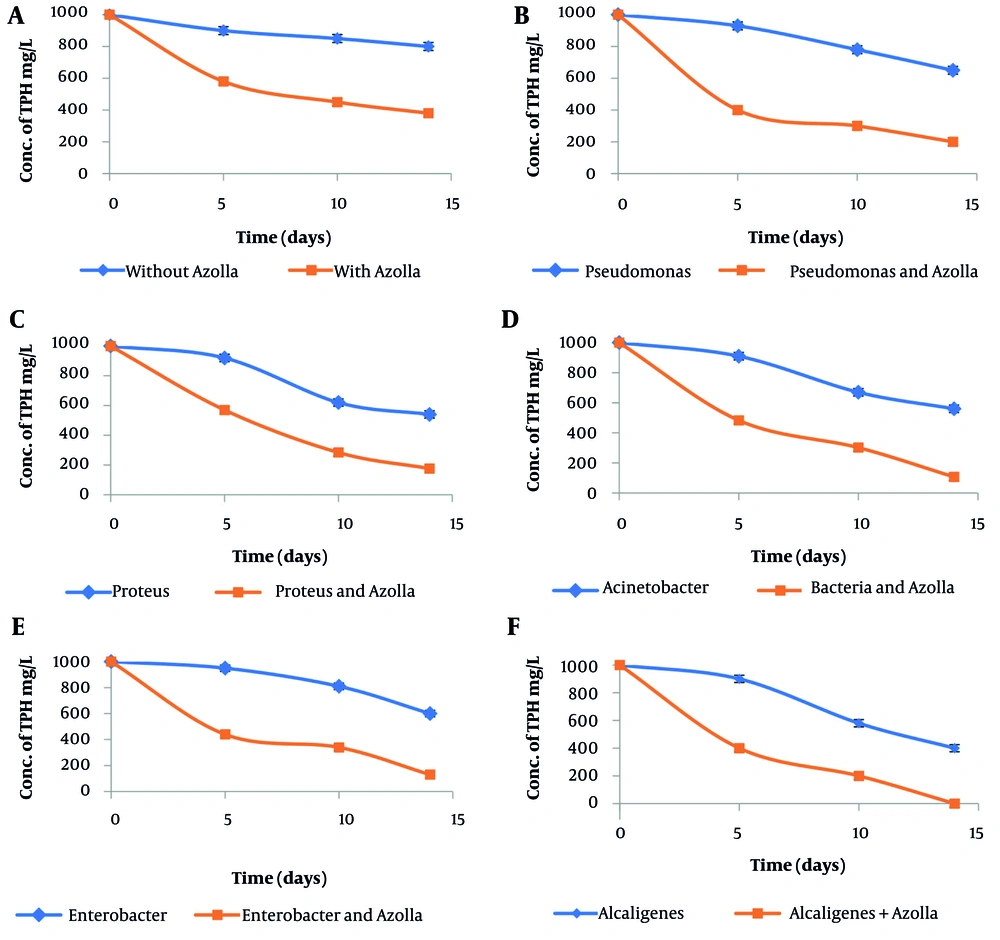
Figure 3 illustrates the removal of TPH at a concentration of 1000 mgL-1 using bacteria and combined Azolla-bacteria culture.
Figure 3A shows that in the control flasks, the concentration of TPH decreased by around 20% after 14 days of contact. Among the examined bacteria, only Alcaligenes was able to remove approximately 60% of TPH (Figure 3F), while the other bacteria showed a removal of around 40% (Figure 3B - E). For all bacteria, the removal of TPH was slow during the first 5 days, but then increased more rapidly. In the combined culture, the highest removal of 100% was achieved by Alcaligenes-Azolla, while the lowest removal of 80% was achieved by Pseudomonas-Azolla.
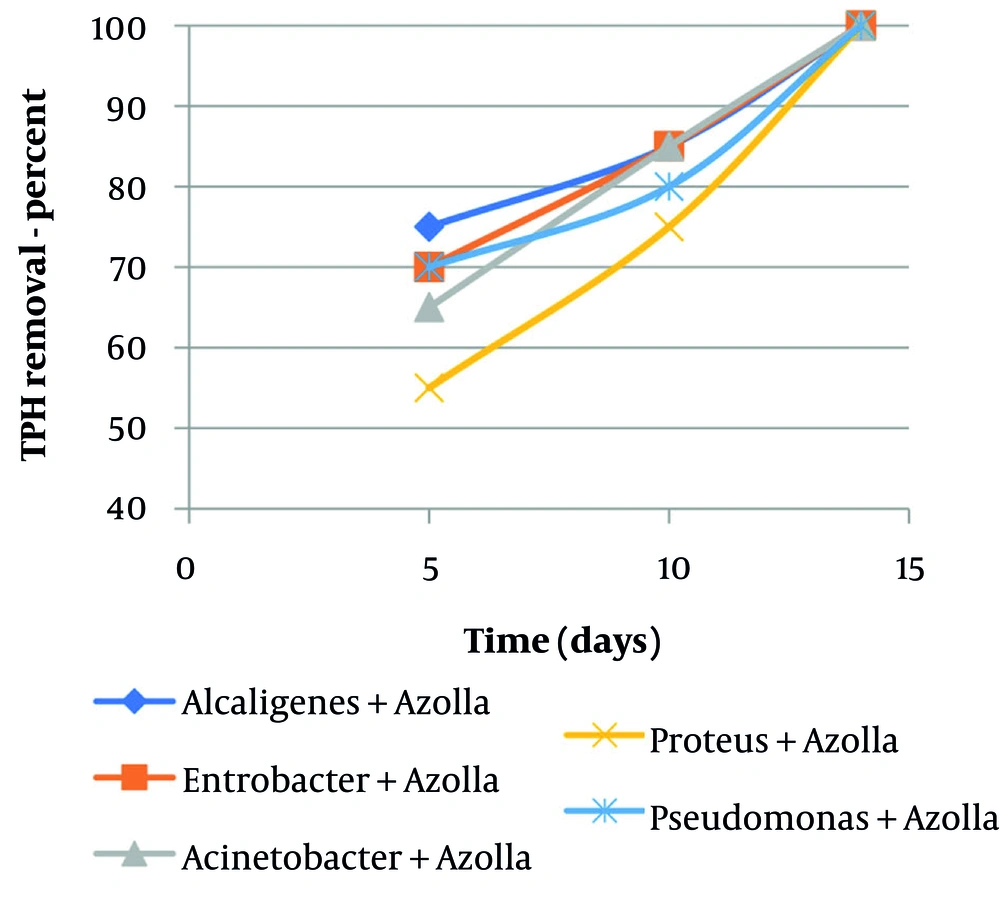
Figure 4 illustrates the removal of TPH from water at a concentration of 500 mgL-1 using a combined culture of bacteria-Azolla. As shown in the figure, the combined culture was able to remove TPH with an efficiency of 85% and 100% after 10 and 14 days, respectively. The lowest removal rate, at 55%, was observed for Pseudomonas-Azolla after 5 days of contact time. The results of all experiments indicate that the removal efficiency of TPH increased as the contact time was extended.
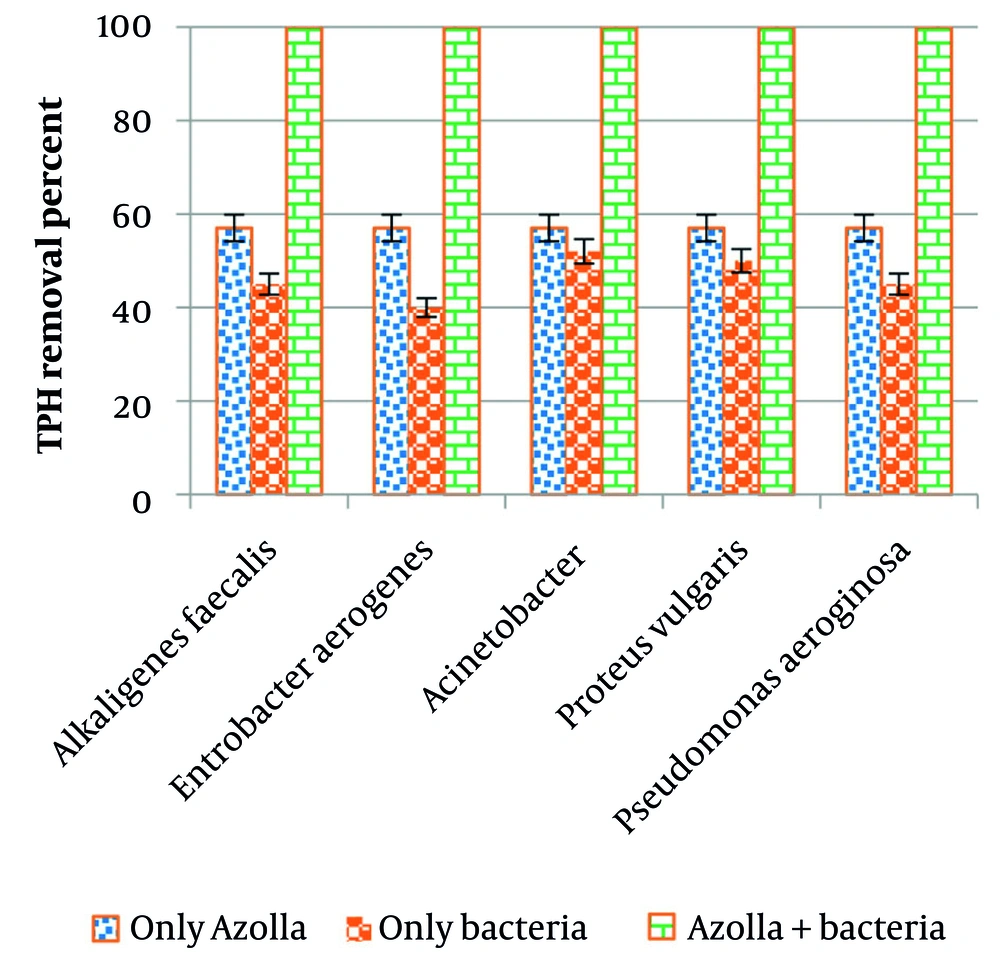
Figure 5 demonstrates that using the combined culture of Azolla-bacteria, 100 mgL-1 of TPH was removed with 100% efficiency within 5 days of contact time. The efficiency of Azolla alone in removing 100 mgL-1 of TPH was 57% within 5 days. The efficiency of each bacterium alone ranged from 40% to 50%, but increased to 100% when using the combined culture of Azolla-bacteria.
5. Discussion
5.1. Identification of Bacteria
The results of the catalase test, which indicate the activity of the catalase enzyme in bacteria, showed gas production in catalase-positive bacteria (30). In the present work, all isolates except P. vulgaris, which exhibited significant hydrocarbon-decomposing ability, were catalase-positive. The oxidase test was used to identify the bacteria that produce cytochrome c oxidase, an enzyme of the bacterial electron transport chain (31). The isolates, P. aeruginosa and Alcaligenes faecalis, were diagnosed as positive oxidase bacteria. The nitrate test is used to determine if an organism is capable of reducing nitrate (NO3-) to nitrite (NO2-) or other nitrogenous compounds via the action of the nitrate reductase enzyme (32). The result of the nitrate reduction test was positive for some species with high hydrocarbon-decomposing ability, including P. vulgaris, Enterobacter aerogenes, and P. aeruginosa.
5.2. Removal of Total Petroleum Hydrocarbon by Azolla
In this study, findings indicate that longer contact times led to higher TPH removal, while higher concentrations of petroleum hydrocarbons led to decreased TPH removal. Observations of Azolla in culture flasks indicated that the withering of Azolla increased with higher concentrations of petroleum hydrocarbons and longer contact times. At a concentration of 1000 mg/L of petroleum hydrocarbons, Azolla was found to be 50% dead. Al-Baldawi et al. studied the exposure of petroleum hydrocarbons on Azolla and found that Azolla was able to survive when exposed to concentrations of 500, 1000, and 3000 ppmv of petroleum hydrocarbons in water for 10 days. However, when exposed to a concentration of 7000 ppmv for 10 days, Azolla completely withered (33).
5.3. Removal of Total Petroleum Hydrocarbon by Bacterial Culture
In this study, for 1000 mgL-1 of TPH, the Alcaligenes bacteria were able to remove 60% of TPH after 14.0 days of contact (Figure 3F). Under the same conditions, the removal of TPH by P. vulgaris and Acinetobacter was 45% (Figure 3 and 3D). TPH removal by Enterobacter and P. aeruginosa was 40% and 35%, respectively Figure 3E and B). In a study, a halotolerant bacterial consortium containing 6 bacterial species, including three strains of P. aeruginosa, removed 83% of crude fuel (34). In research, the decomposition of 3% vol. of crude fuel in culture medium by P. aeruginosa was 60% (35).
5.4. Removal of Total Petroleum Hydrocarbon by Combined Culture of Azolla and Bacteria
The data from the graphs in Figure 3 demonstrate that the amount of TPH removal in the Azolla-bacteria combined culture is 40 - 45% higher than in the pure bacterial culture. The bioremediation of petroleum hydrocarbons using plant-microbe systems has been studied, and it has been reported that the compounds identified around the Azolla roots are similar in molecular structure to the aromatic compounds found in petroleum hydrocarbons. Additionally, Azolla has been shown to aid in the formation of microbial flocs by providing nitrogen for the degradation of petroleum hydrocarbons (12).
5.5. Mechanisms of Total Petroleum Hydrocarbon Degradation by Bacteria
Biodegradation of petroleum hydrocarbons by bacteria depends on many factors, which are briefly described. One factor is the bioavailability and bioaccessibility of petroleum hydrocarbons for bacteria. Bioavailability is defined as the fraction of the total amount of any contaminant or pollutant present in the environment that has the potential to be absorbed by the organism, and bioaccessibility refers to the total amount of substrate that the organism can uptake (36). Microbial adhesion to hydrophobic surfaces, which is the transfer of suspended cells from the aqueous phase to the water-oil interface, is a mechanism that occurs when the bioavailability of the substrate is low (37). The presence of biosurfactants affects the adhesion of bacteria to oil droplets by changing the hydrophobicity of the cell wall (38).
5.6. Conclusions
Removing a thin layer of petroleum hydrocarbons from the surface of water by physico-chemical methods is not cost-effective. A combined plant-bacteria bioremediation method using a floating plant like Azolla could be a suitable option for removing petroleum hydrocarbons from water. Removal of 100, 500, and 1000 mgL-1 of TPH from water was studied by co-culture of Azolla and bacteria and compared to Azolla and bacteria cultures alone. Results show that complete removal (100%) of TPH (concentration of 100 and 500 mgL-1) occurred using the co-culture method.
By releasing substances such as sugar compounds, enzymes, and oxygen from the roots, as well as providing a contact surface for the formation of bacterial biofilm, the plant can attract bacteria to the root areas. These bacteria can establish mutual relationships with the plant, increase their survival, and decompose toxic substances in water or soil. The strength of this study is the improvement of the efficiency of bioremediation of petroleum hydrocarbons from water using the co-culture method of Azolla-bacteria. The limitation of this study is that the work was done on a laboratory scale. It is recommended that further studies be conducted in this field under real environmental conditions so that this method can be used on a real scale to remove residual petroleum hydrocarbons in water.