1. Background
Blood donor selection is an essential part of blood safety. Therefore, uniform questionnaire as well as deferral criteria should be used to assess the suitability of volunteers (1-3). Using national standards, international guidelines, and safety concerns, the Iranian blood transfusion organization (IBTO) has established donor suitability criteria and deferral policies to ensure the effectiveness of donor selection (4, 5).
The first and the most important step ensuring high levels of blood component safety is establishing an appropriate donor selection (6-9), which ensure that the effectiveness of donor selection by employing a trained and qualified physician as a blood transfusion specialist, using a private interview room; donors shall be deferred based on national donor deferral criteria, completion of uniform donor questionnaire, and evaluation by national deferral registry software across the country. In addition to the abovementioned procedures, IBTO has adopted other steps, such as self-deferral procedure, confidential unit exclusion, call-back, recall, look back, increase regular donation, and pre-donation laboratory screening to assure the health and usability of the blood that is delivered to hospitals and medical centers (6, 10-13). Based on these principles, 21.6% of 2,552,084 volunteers who had referred to 91 blood transfusion centers across the country in 2019 were deferred from blood donation (unpublished data from the national database on blood donors).
Some studies that have assessed the effectiveness of the donor selection process by the correlation between the potential donors who were rejected due to having high-risk behaviors and the prevalence of transfusion-transmitted infections. A study by Zou et al. (2006) reported that donors deferred for a history of intravenous drug use were more likely to have hepatitis infection than those who were eligible to donate blood. Since the sample size of this study was too small to find any HIV-positive donor, it does not have enough power to discuss this factor (8). In a study from Brazil that is conducted between September 2010 to March 2011 on participants deferred because of high-risk behaviors, the authors reported that the highest frequency of HIV and Syphilis tests was among TTI, indicating that the deferral questions exclude cases with higher risks for these infections and there was no significant difference between HBV and HCV comparing deferred and first-time donor prevalence (13). An earlier study in Iran has compared the prevalence of HIV, HBV, and HCV markers among deferred and eligible blood donors for evaluating the efficacy of the current donor selection procedure. This study showed obvious effectiveness and the requirement of donor selection however the data were not analyzed according to the deferral reason. Also, a detailed description of the eligible group in the indicated period of time was not provided (3).
2. Objectives
Based on what was mentioned before, we evaluated and monitored the efficacy of the donor selection process, by comparing the prevalence of HBV, HCV, and HIV infection among eligible blood donors and blood donor volunteers who were deferred due to high-risk behaviors.
3. Methods
3.1. Study Population and Data Collection
The current research was performed on 2,525 high-risk deferred donors who were presented for blood donation from March 1, 2018, to September 30, 2019, at the twenty participating blood transfusion centers in IBTO. The sample size was calculated based on a previous study conducted in Iran by Razjou et al (3). Apart from collecting characteristics of deferral donors (such as age, sex, occupation, marital status, education, and history of donation (first-time, repeat, and regular)), the method of sampling was convenience as if the volunteers were interviewed by a physician and differed from donation if they don’t meet any blood donor selection criteria including: History of current or past viral infection, injecting or non-injected drugs use, blood transfusions, unsafe current sexual, and household contact of individuals with an active infection, cosmetic treatments, and rituals (tattoo, Hijama, Acupuncture), history of detention in jail for more than 72 hours, and a history of medical and surgical interventions by using a uniform questionnaire and the participants signed the informed consent. To make a better prospect of the results, number and percentage donors' demographics description and TTI marker rates of 1,315,871 eligible donors in the indicated period obtained from the national database on blood donors totally. The ethics committee of the high institute of research & education in transfusion medicine approved the research.
3.2. Laboratory Analyses
A blood sample was collected from all enrolled deferred donors. All participants and eligible blood donors were evaluated regarding serologic 3 viral markers, including HBsAg (Monalisa HBs Ag ULTRA, Bio-Rad France), HCV Ab (Monalisa Anti-HCV PLUS Version 3, Bio-Rad France), and HIV Ag/Ab (Genscreen ULTRA HIV Ag/Ab, Bio-Rad France) by ELISA based on the instruction of test kits. The initially reactive samples were tested duplicate. All repeatedly reactive specimens were confirmed by Hepatitis B core antibody (Enzygnost Anti-HBc monoclonal, Siemens Germany) and HBs Ag confirmatory test (Enzygnost HBs Ag confirmatory test, Siemens Germany), HCV Blot (INNO-LIA HCV Score, Fujirebio Europe NV) and HIV Western Blots (INNO-LIA HIV I/II Score, Fujirebio Europe NV).
3.3. Statistical Analysis
Data were analyzed using SPSS version 24.0. For assessing frequency of characteristics variables the prevalence of variables per 100 000 donations and 95% confidential intervals (95% CIs) was calculated. The odds ratio (OR) of the association between deferral due to a history of high-risk behaviors and viral infections was determined. All reported p-values were two-sided. A p-value of < 0.05 was regarded significant.
4. Results
4.1. Characteristics of Enrolled Deferred and Eligible Blood Donors
A total of 2,525 high-risk deferred blood donors in the period of March 2018 - September 2019 were enrolled as participants. The majority of deferred blood donors due to high-risk behaviors who participated in the study were male first-time donors (n = 1129, 44.7%).
The total number of eligible blood donors was 1,315,871. Most of the participants of the eligible donors group were regular donors (58.1%), while in the deferred group, 27.3 % of participants were regular donors. Besides, in the eligible donor group, 1,065,040 (81%) were married, so that the percentage of married donors was about 4 times of single donors and 1.7 times of married donors in the deferred group.
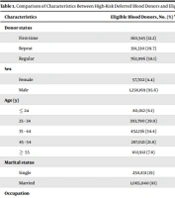
Details of characteristics of high-risk deferred blood donors and eligible blood donors are presented in Table 1.
| Characteristics | Eligible Blood Donors, No. (%) a | High-Risk Deferred Blood Donors, No. (%) | P Value |
|---|---|---|---|
| Donor status | < 0.001 | ||
| First-time | 160,545 (12.2) | 1,349 (53.4) | |
| Repeat | 391,330 (29.7) | 488 (19.3) | |
| Regular | 763,996 (58.1) | 688 (27.3) | |
| Sex | < 0.001 | ||
| Female | 57,702 (4.4) | 356 (14.1) | |
| Male | 1,258,169 (95.6) | 2,169 (85.9) | |
| Age (y) | < 0.001 | ||
| ≤ 24 | 80,012 (6.1) | 587 (23.2) | |
| 25 - 34 | 393,790 (29.9) | 1,057 (41.9) | |
| 35 - 44 | 452,136 (34.4) | 492 (19.5) | |
| 45 - 54 | 287,021 (21.8) | 302 (11.9) | |
| ≥ 55 | 102,912 (7.8) | 87 (3.5) | |
| Marital status | < 0.001 | ||
| Single | 250,831 (19) | 1,304 (52) | |
| Married | 1,065,040 (81) | 1,221(48) | |
| Occupation | < 0.001 | ||
| Government's employee | 374,482 (28.4) | 533 (21.1) | |
| Non-government employee | 697,104 (53) | 1,396 (55.3) | |
| Unemployed | 20,964 (1.6) | 101 (4.0) | |
| Student | 68,023 (5.2) | 21 (8.4) | |
| Housewife | 39,005 (3) | 258 (10.2) | |
| Others | 116,293 (8.8) | 24 (1) | |
| Education | < 0.001 | ||
| Less than diploma | 341,309 )26) | 664 (26.3) | |
| Diploma b | 582,216 (44.2) | 1199 (47.5) | |
| University degree | 392,346 (29.8) | 662 (26.2) | |
| Total | 1,315,871 | 2,525 |
aThis data was obtained from national database on blood donors.
bA certificate awarded by Iranian education system to show that someone has successfully completed high school.
Results of TTIs screening tests (HBV, HCV, HIV) in enrolled deferred and eligible blood donors
To provide a better perspective of the results and to shed more light on the efficiency of IBTO screenings, we compared the results of deferred and eligible blood donors. Notably, according to the findings, the prevalence of TTIs was significantly higher among the deferred group as compared with eligible donors.
The overall prevalence of infections in deferred donors group compared to eligible donors group was 1.78% to 0.07% (a difference equal to 25.4 times) (table 2). Expression of this ratio separately for tests revealed the following results: HBV Odds Ratio = 26.30 (95% CI: 18.1 - 38.3), HCV Odds Ratio = 28.02 (95% CI: 16 - 49), and HIV Odds Ratio = 33.30 (95% CI: 10.4 - 107.1). Since the proportion of first-time donors among deferred donors was higher than the eligible ones, we described OR as follows: HBV Odds Ratio = 4.34 (95% CI: 2.7 - 6.9), HCV Odds Ratio = 6.83 (95% CI: 3.8 - 12.2), and HIV Odds Ratio = 7.94 (95% CI: 1.0 - 60.1).
| Characteristics | Eligible Blood Donors (N = 1,315,871) b | High-Risk Deferred Blood Donors, (N = 2,525) | P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HBV Positive | HCV Positive | HIV Positive | HBV Positive | HCV Positive | HIV Positive | HBV | HCV | HIV | |
| Donor status | |||||||||
| First-time | 527 (328) | 212 (132) | 15 (9) | 19 (1408) | 12 (889.5) | 1 (74) | < 0.001 | < 0.001 | NS |
| Repeat, | 27 (7) | 23 (6) | 20 (5) | 9 (1844) | 1 (205) | 1 (205) | < 0.001 | 0.029 | 0.026 |
| Regular | 27 (3.5) | 8 (1) | 12 (1.5) | 1 (145) | 0 (0) | 1 (145) | 0.025 | 0.012 | |
| Sex | |||||||||
| Female | 40 (69) | 11 (19) | 1 (2) | 2 (561) | 0 (0) | 0 (0) | 0.027 | ||
| Male | 541 (43) | 232 (18) | 46 (4) | 27 (1244) | 13 (599) | 3 (138) | < 0.001 | < 0.001 | < 0.001 |
| Age (y) | |||||||||
| ≤ 24 | 13 (16) | 5 (6) | 1 (1) | 1 (170) | 0 (0) | 0 (0) | NS | ||
| 25 - 34 | 171 (43) | 63 (16) | 11 (3) | 9 (851) | 6 (568) | 0 (0) | < 0.001 | < 0.001 | |
| 35 - 44 | 197 (43.5) | 118 (26) | 16 (3.5) | 11 (2235) | 3 (610) | 2 (406.5) | < 0.001 | < 0.001 | < 0.001 |
| 45 - 54 | 139 (48) | 49 (17) | 19 (7) | 6 (1986) | 3 (993) | 1 (331) | < 0.001 | < 0.001 | 0.021 |
| ≥ 55 | 61 (59) | 8 (8) | 0 (0) | 2 (2298) | 1 (1149) | 0 (0) | < 0.001 | 0.008 | |
| Marital status | |||||||||
| Single | 65 (26) | 68 (27) | 11 (4) | 3 (230) | 7 (536) | 1 (77) | 0.005 | < 0.001 | NS |
| Married | 516 (48) | 175 (16) | 36 (3) | 26 (2129) | 6 (491) | 2 (164) | < 0.001 | < 0.001 | < 0.001 |
| Occupation | |||||||||
| Government's employee | 120 (32) | 38 (10) | 9 (2) | 9 (1688) | 2 (375) | 0 (0) | < 0.001 | 0.002 | |
| Non-government employee | 333 (48) | 144(21) | 28(4) | 19(1361) | 11(788) | 3(215) | < 0.001 | < 0.001 | < 0.001 |
| Unemployed | 10 (48) | 6 (29) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Student | 4 (6) | 1 (1) | 3 (4) | 0 (0) | 0 (0) | 0 (0) | |||
| Housewife | 27 (69) | 11 (28) | 1 (2.5) | 1 (387.5) | 0 (0) | 0 (0) | NS | ||
| Others | 87 (75) | 41 (35) | 8 (7) | 0 (0) | 0 (0) | 0 (0) | |||
| Education | |||||||||
| Less than diploma | 280 (82) | 121 (6) | 16 (5) | 13 (1957) | 9 (1355) | 2 (301) | < 0.001 | < 0.001 | < 0.001 |
| Diploma c | 216 (37) | 92 (16) | 21 ( 4) | 13 (1084) | 3 (250) | 1 (83) | < 0.001 | < 0.001 | 0.044 |
| University degree | 85 (22) | 37 (9) | 13 (3) | 3 (453) | 1 (151) | 0 (0) | < 0.001 | NS | |
| Total | 581 (44) | 243 (18 .5) | 47 (3.6) | 29 (1148) | 13 (515) | 3 (119) | < 0.001 | < 0.001 | < 0.001 |
Abbreviation: NS, Not Significant.
aValues are expressed as No. (Prevalence Per 105) unless otherwise indicated.
bThis data was obtained from national database on blood donors
cDiploma, A certificate awarded by Iranian education system to show that someone has successfully completed high school.
As well, the proportion (%) of identified TTIs among male deferred blood donors vs. females is 1.98% to 0.56 (a difference equal to 3.5 times), but in eligible group, it was vice versa, in such a way that this ratio was 0.06 to 0.09. Among deferred donors, the positive TTI among married donors was 3.5 times higher than single people.
A detailed analysis of the characteristics of eligible donors and high-risk deferred blood donors concerning HBV, HCV, and HIV infections is summarized in Table 2.
5. Discussion
Few studies have evaluated the effectiveness of specific donor deferrals on blood safety (14). Hence, it was of particular interest to evaluate whether the present screening process in Iran could precisely eliminate the infected blood from the transfusion cycle. According to the results, a total of 871 of 1,315,871 (0.07%) eligible donors and 45 of 2,525 (1.78%) high-risk deferred donors were positive for one TTI. In total, confirmed infections among eligible donors were as follow: HBV (n = 581), HCV (n = 243) and HIV (n = 47). Besides, a concerning deferred donors was identified: HBV (n = 29), HCV (n = 13) and HIV (n = 3); (Table 2). This study introduced HBV as the major cause of deferral due to TTI in deferred (1.14%) and eligible groups (0.04%) as well as the leading cause of deferral followed by HCV and HIV. Although the results of other studies suggested HBV as the main reason for blood deferral (3, 15), a study performed in Brazil in 2010 - 2011 reported that HIV plays a significant role among behavioral deferrals (13). Also, another study in America has reported a high prevalence of HCV among allogeneic donations (16). This difference in the prevalence of TTIs may be due to geographical differences and social habits of individuals.
During the study period, the likelihood of HCV and HIV infection was nearly 28 and 33 times more likely in deferred compared to eligible donors. Another study conducted in Iran reported a similar ratio in HBV (1.3% and 0.7%), HCV (0.6% and 0.1%), and HIV (0.1% and 0.005%) in deferred and eligible groups (3, 7). Monitoring of TTIs in the current study and that conducted by Razijo in Iran indicates two points: 1) a significant reduction in the prevalence of HBV, HCV, and HIV among eligible donors over time (2012 to 2019); and 2) an upward trend in the prevalence of viral infections in deferred compared to eligible donors (3) .It should be noted that the previous study did not analyze the data based on deferral due to high-risk behavior, and that's why the ratio of positive cases in deferred groups is only 2-times more than eligible donors, while in the present study, this ratio is about 25.4-times. According to another study in a city of Iran (Ahwaz), the HBV, HCV, and HIV rates in deferred and eligible blood donors is estimated as (0.5% and 0.2%), (1.3%and 0), and (0.2% and 0.05%), respectively (11). Generally, analyses demonstrated that the prevalence of the TTI marker among the deferred group was higher as compared to the eligible group in Iran, highlighting the efficacy of the donor screening process and pre-donation deferral criteria in exclusion of individuals with high risks of infection. This finding is confirmed with the results obtained by a study conducted in Australia, which demonstrated a reduction in the prevalence of screened TTIs in all eligible donors by a factor of 50 to 350, compared with the general population (17).
Another study conducted in Senegal confirmed that medical screening questions are efficient for preventing blood donors at high risk of HIV (1.75% vs. 0.05%) transmission as well as for a lesser extent of HBV (12.87% vs. 7.35%) (18, 19). In contrast, Zou in an American Red Cross study showed that donors deferred for bloodborne pathogen risk (BBPR) who returned did not show a higher risk of viral infections under study. This result can be attributed to the fact that those who were initially deferred and referred for another time and were included in the study had lower risks of infection with viral agents (20-22).
Our results showed a significantly higher prevalence of HCV, and HBV (6.7 and 4.3-fold) in the deferred first-time donor compared with the eligible first-time donors. Although the overall prevalence of TTI in first-time donors of the two group may be different in studies, an American study by Gonçalez showed the prevalence of HIV (0.35% vs. 0.092%) and syphilis (2.81% vs. 0.54%) in deferred donors was significantly more compared with first-time eligible donors. Nonetheless, for HCV and HBV infection, this study reported a similar prevalence in both groups, which suggests that their questionnaire was probably well-screened concerning HIV and syphilis cases (13).
The ratio of infection among first-time donors versus repeat and regular donors in the eligible group was 31.2:1 (HBV), 18.8:1 (HCV), and 1.4:1 (HIV). In a study by Zou et al., a similar ratio is reported for HBV (112.2:1), HCV (35.4 :1), and HIV (7.3:1) (16). These higher probability rates of infections among first-time donors as compared to repeat and regular donors and the fact that first-time donors constituted the majority of the deferred cases (53.4%) (23), while the regular donors constituted the majority of eligible donors (58.1%) (consistent with the results reported by Gonçalez) (13, 24) can be attributed to the sensitivity of regular volunteer donors about their blood safety, because they do not have high-risk behaviors such as unsafe current sex or intravenous drug abuse. we concluded that regular donors are safer than first-time donors concerning the safety of blood donation, which is why IBTO recommends encouraging regular donors to donate blood (23).
The majority of 2,525 deferred donors and 1,315,871 eligible donors were male 85.9 and 95.6%, respectively, which is in concordance with published studies (3, 11, 13, 15, 24). While in the deferred group, the prevalence of HBV among males was almost twice of females. In the eligible group, the prevalence of HBV and HCV in females was more in comparison with males. Zou et al. reported that the prevalence ratio between males and females for HBV, HCV, and HIV among allogeneic donations was 1.63, 1.19, and 2.99, respectively (16). The overall prevalence of HIV in both groups was higher among males as compared to females, which is in agreement with the results of Bartonjo and colleagues (15). The results suggested that in the deferred group the deferration rate was decreasing and the maximum deferration was under 34 years (similar to the results reported by Gonçalez) (13). The prevalence proportion of TTIs was increasing with age (0.17% to 3.45%). The marital status stratification of the deferred donors showed that the deferral rate of singles is 2.7 times higher than singles in the eligible group (52% vs 19%, respectively). Besides, the singles had an odds of 4.5 against 1 (95%CI = 4.2 - 4.9) for being deferred. It seems that married people (81%) are more likely to gain the trust of the blood transfusion organization in the selection process because they are more likely to keep away from risky behaviors. But caution should be taken, as in this study, we found a high prevalence of hepatitis B in married participants of both groups (similar to the results of Bartonjo) (15). Also, the prevalence of HIV was significantly higher among married participants of the deferred group.
In the current study, no substantial difference was observed concerning the education rates of eligible and deferred donors. Diploma (a certificate awarded by Iranian education system to show that someone has successfully completed high school) are the most likely participants in the donation and the prevalence of infection is reduced by university degree in both groups, so it is safer to supply blood to people with higher education. (similar to the results reported by Razjou F) (3).
It is recommended to use nucleic acid test (NAT) in deferred donors due to their high-risk behaviors in parallel with serology screening, (6, 25, 26).
5.1. Conclusion
In conclusion, we believe due to epidemiological, demographic, and even cultural changes in each country, this process should be continuously evaluated and reviewed, if necessary. Our results highlighted that the accuracy of current deferral criteria and donor selection procedure in Iran is an opportunity to eliminate high-risk individuals from the blood donation and play an effective role for blood safety improvement.