1. Background
Hepatic fibrosis in chronic liver disease is characterized by excessive collagen deposition and is a major public health concern (1, 2). During liver fibrogenesis, activation of hepatic stellate cells (HSCs) initiates processes leading to liver injury (3). Hepatic stellate cells are liver-specific pericytes that transform into myofibroblasts, which are involved in pathological vascularization in liver fibrosis (4). Previous studies have shown that hepatic inflammation is tightly associated with fibrosis (5).
Quiescent HSCs are vitamin A-storing cells located in the perisinusoidal space near the sinusoid endothelial cells (SEC) and hepatocytes (6). Hepatic stellate cells can become activated due to chemokines and cytokines released during liver injury. Activating signals include platelet-derived growth factors (PDGF), transforming growth factor-β (TGF-β), and various cytokines, such as interleukin-6 (IL-6) (7). The continuous activation of HSCs can secret extracellular matrix (ECM) such as α-smooth muscle actin (α-SMA), collagens, and inflammatory mediators, which contribute to hepatic inflammation and fibrosis (8). Thus, it is important to suppress hepatic inflammation in the initial stages of chronic liver diseases (7). Blocking or retarding the excessive inflammatory process could be a major therapeutic target for preventing hepatic fibrosis.
Zinc finger protein A20 has been found to negatively regulate inflammation with crucial physiological functions that protect the liver (9, 10). Lipopolysaccharide (LPS) can induce severe inflammation and enhance fibrosis in liver damage models (11). Treating human HSCs with LPS dramatically activates the inflammatory signaling pathway and aggravates the accumulation of proinflammatory chemokines (6). A20 is an intracellular ubiquitin-editing enzyme that has been shown as a crucial hepatoprotective factor to prevent chronic liver inflammation (12). A20 plays an important role in liver protection through limiting inflammation following nonalcoholic fatty liver disease (NAFLD), chronic liver allograft dysfunction, hepatic ischemia-reperfusion injury, and acute liver failure (ALF) (10, 13-16). We previously suggested that A20 overexpression suppresses LPS-induced inflammation in HSC (17). However, the mechanisms through which A20 reduces inflammation in HSCs are unclear. Here, we evaluated the mechanisms of A20 anti-inflammatory effects in LX-2, a HSC cell line.
2. Objectives
In this study, we aimed to determine the mechanisms of the anti-inflammatory role of A20 in LX-2 cells via A20 overexpression and A20 knockdown.
3.Methods
3.1. Ethical Approval Certificate
The ethics committee of the hospital to Jiaxing University approved this study (No.2017-002). The study adhered to 1964 Helsinki Declaration guidelines.
3.2. Cell Culture and Treatment
LX-2 cells were obtained from ATCC and grown in DMEM (Gibco, Cat No.670087), containing 1% pen/strep and 10% FBS at 37°C in a humidified incubator, 5% CO2. E. coli O055:B5 LPS (Sigma, Cat No.L6529) was used to stimulate the LX-2 cells at 0.1 μg/mL,, according to our previous research (17), for indicated durations before they were harvested and washed at least three times with PBS 1X for analysis.
3.3. Design and Transfection of Adenovirus
The control adenovirus and A20 overexpression adenovirus (Ad-A20) were designed by Obio Technology (shanghai) Co. Ltd. Shanghai, China. The adenovirus was produced and tittered as described (18). The two adenovirus preparations were transfected into LX-2 cells for 24 h. The media were replaced with fresh media, and cells grown to 80 - 90% confluence. They were then rinsed with PBS 1X and cultured with serum-free DMEM/F12 before stimulation with LPS for indicated durations. Cells were then harvested and cryopreserved for later use.
3.4. Design and Transfection of siRNA
A20-siRNA (forward 5’-UAAGAUUGUCCCAUUCAUCTT-3’, reverse 5’-GAUGAAUGGGACAAUCUUATT-3’) and control siRNA (forward 5’-UUCUCCGAACGUGUCACGUTT-3’, reverse 5’-ACGUGACACGUUCGGAGAATT -3’) were designed by KeyGEN BioTechnology Co. Ltd. Nanjing, China. Lipofectamine 3000 (Invitrogen, CA, USA) was used to transfect control siRNA and A20-siRNA into LX-2 cells in 6-well cell culture plates. The concentration of control siRNA and A20-siRNA was 100 nM. The media were replaced with fresh media after 4 - 6 h of transfection, and cells were grown to 80-90% confluence. They were then rinsed with PBS 1X and cultured with serum-free DMEM/F12 before stimulation with LPS for indicated durations. Cells were then harvested and cryopreserved for later use.
3.5. Western Blotting
The cell samples were washed with PBS and digested with 1% Triton X-100 supplemented with protease inhibitors, 150 mM NaCl, and 10% glycerol for 30 min at 4°C. Lysates were cleared by centrifugation at 4°C for 15 min. The quantity of the extracted proteins was obtained using bicinchoninic acid (BCA) assay (Thermo Fisher Scientific, Cat No.23225). Next, 30 μg of protein samples was resolved on SDS-polyacrylamide gel and electrotransferred onto nitrocellulose membranes (Pall, Cat No.66485) before probing with anti A20 (1:1000), ERK (1:1000), phosphorylated ERK (1:400), JNK (1:1000), and phosphorylated JNK (1:1000) primary antibodies obtained from Cell Signaling Technology. The membranes were incubated with primary antibodies at 4°C overnight.
After washing in TBST three times, the samples were treated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit or mouse IgG for 1 hour at room temperature. Blots were visualized by using enhanced chemiluminescence (ECL) kit (Millipore, MA, USA) and detected by the G:BOX chemiXR5 imager. The intensities of the protein bands were quantified by ImageJ software and calculated as a ratio of phosphorylated ERK and phosphorylated JNK to total ERK and JNK. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the loading control.
3.6. RNA Extraction and RT-qPCR Analysis
Cells were treated with Trizol to extract RNA, which was reverse-transcribed at 37°C for 15 min and at 85°C for 5s in a 20 μL reaction volume with PrimeScript™ RT reagent Kit (Takara, Cat No.RR037A) following manufacturer instructions. Primers are presented in Table 1. For all the qRT-PCR experiments, primer efficiency was verified to be greater than 95%. Quantitative reverse transcription PCR (RT-qPCR) assay was performed on a StepOnePlus™ Real-Time PCR system at 95°C for 5 min, followed by 40 cycles at 95°C for 15s and at 60°C for 20s in a 20-μL reaction volume, as described in the manufacturer’s guidelines on a StepOnePlus™ Real-Time PCR System by Applied Biosystems (Applied Biosystems Inc., California, USA). Relative mRNA levels for the target genes were obtained by the 2(-ΔΔCt) method with GAPDH serving as the house-keeping gene.
| Primer | Sequence |
|---|---|
| α-SMA | |
| Forward | 5’- ACTGCCTTGGTGTGTGACAA -3’ |
| Reverse | 5’- CACCATCACCCCCTGATGTC -3’ |
| Col-I | |
| Forward | 5’- GCTCGTGGAAATGATGGTGC -3’ |
| Reverse | 5’- ACCCTGGGGACCTTCAGAG -3’ |
| Col-III | |
| Forward | 5’- TGCCCTACTGGTCCTCAGAACT -3’ |
| Reverse | 5’- CCTGCGAGTCCTCCTACTGCTA -3’ |
| TGF-β | |
| Forward | 5’- AGGACCTCGGCTGGAAGTGGAT -3’ |
| Reverse | 5’- AGGACCTTGCTGTACTGCGTGT -3’ |
| IL-6 | |
| Forward | 5’- CCTTCGGTCCAGTTGCCTTCTC -3’ |
| Reverse | 5’- AGAGGTGAGTGGCTGTCTGTGT -3’ |
| PDGF | |
| Forward | 5’- GCTTGGCTCGTGGAAGAAGGAG -3’ |
| Reverse | 5’- TTGGCGTTGGTGCGGTCTATG -3’ |
| GAPDH | |
| Forward | 5’- CAAATTCCATGGCACCGTCA -3’ |
| Reverse | 5’- AGCATCGCCCCACTTGATTT -3’ |
Primer Sequences
3.7. Statistical Analysis
All the data were analyzed using Graphpad Prism 5 (Graphpad, San Diego, CA) and are shown as mean ± SD of three independent experiments. The mean values of different groups were compared using ANOVA or independent samples t-test. A P-value of less than 0.05 indicates statistical significance.
4. Results
4.1. A20 Suppresses LPS-induced Fibrosis-Related Molecules and Inflammatory Response in LX-2 Cells
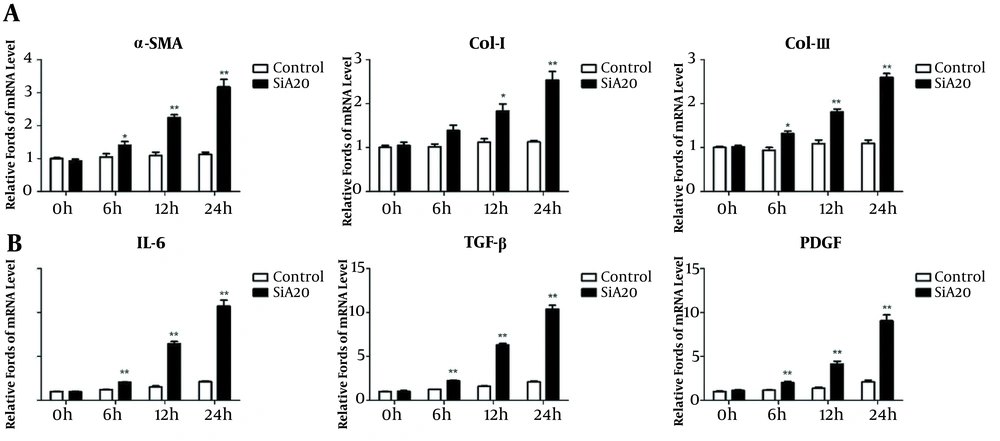
Using immunohistochemistry, we previously observed elevated A20 levels in hepatic fibrosis patients (17). Here, we examined fibrosis-related molecules and inflammatory cytokine expression in control and A20-siRNA cells. Relative to controls, the fibrosis-related mRNA levels of α-SMA, col-I, and col-III were increased in LX-2 cells transfected with A20-siRNA (Figure 1A). A20-siRNA cells significantly increased IL-6, TGF-β, and PDGF mRNA levels (Figure 1B), which activated HSCs (19), suggesting that A20 regulates these effectors of HSCs activation.
A20 knockdown (siRNA) promotes the mRNA expression of fibrotic markers and LPS-induced inflammatory response in LX-2 cells. A, The mRNA expression of α-SMA, col-I, and col-III in LX-2 cells transfected with A20-siRNA and control exposed to various times of LPS. B, mRNA levels of IL-6, TGF-β, and PDGF in LX-2 cells transfected with A20-siRNA and control exposed to various times of LPS. GAPDH was used as the reference gene. * P < 0.05 and ** P < 0.01 relative to control groups.
4.2. MAPK/ERK/JNK Pathway Levels Are Elevated in LX-2 Cells in Response to LPS
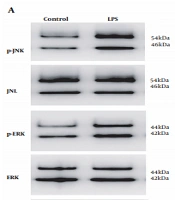
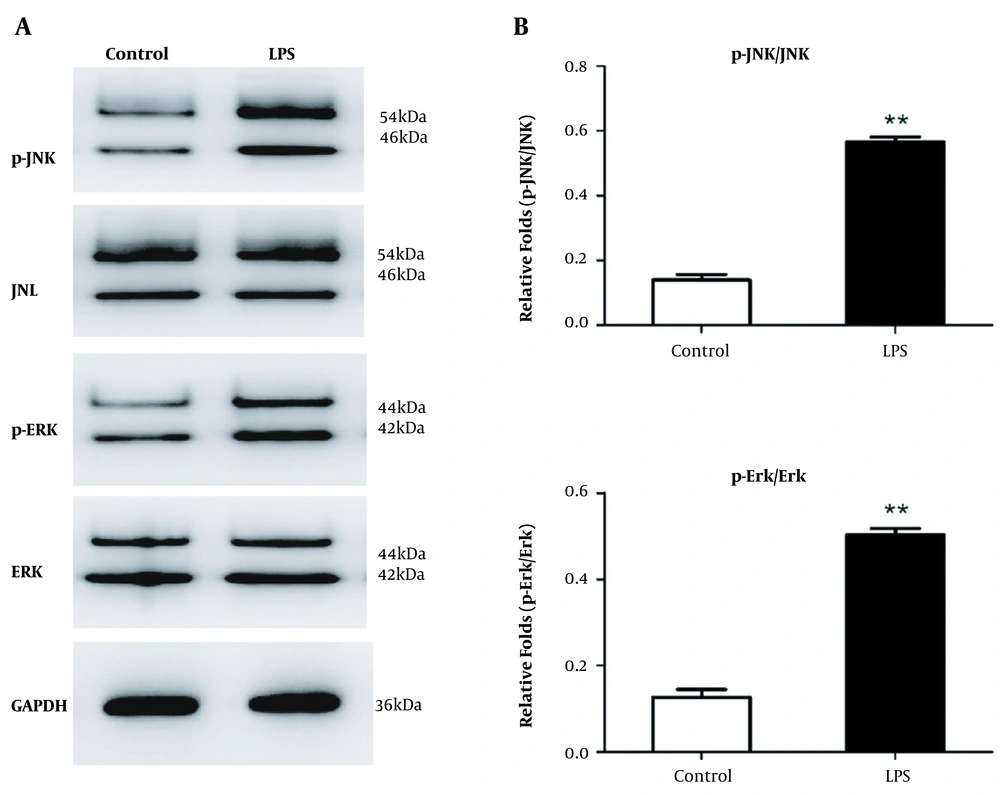
We previously found that LX-2 cells mount an inflammatory response and upregulate fibrosis indicators in response to LPS. Here, we focused on the MAPK/ERK/JNK signaling, which controls various processes in HSCs (20-22). Western blot analysis revealed elevated phosphorylated JNK and phosphorylated ERK levels in LX-2 cells relative to control (Figure 2). In order to explore whether A20 expression was involved in MAPK/ERK/JNK pathway, we overexpressed A20 via Ad-A20 and silenced A20 via A20-siRNA targeting A20 in LX-2 cells to analyze the changes.
MAPK/ERK/JNK pathway levels are elevated in LX-2 cells. A, Western blot analysis of MAPK/ERK/JNK levels in LX-2 cells in response to LPS for 30 min. GAPDH served as the gene for loading control. B, Gray analysis of relative fold changes of protein phosphorylated JNK (p-JNK)/JNK and phosphorylated ERK (p-ERK)/ERK. P-JNK, JNK, p-ERK, and ERK represented as the sum of two bands. * P < 0.05 relative to control groups.
4.3. Function of A20 in MAPK/ERK/JNK Pathway
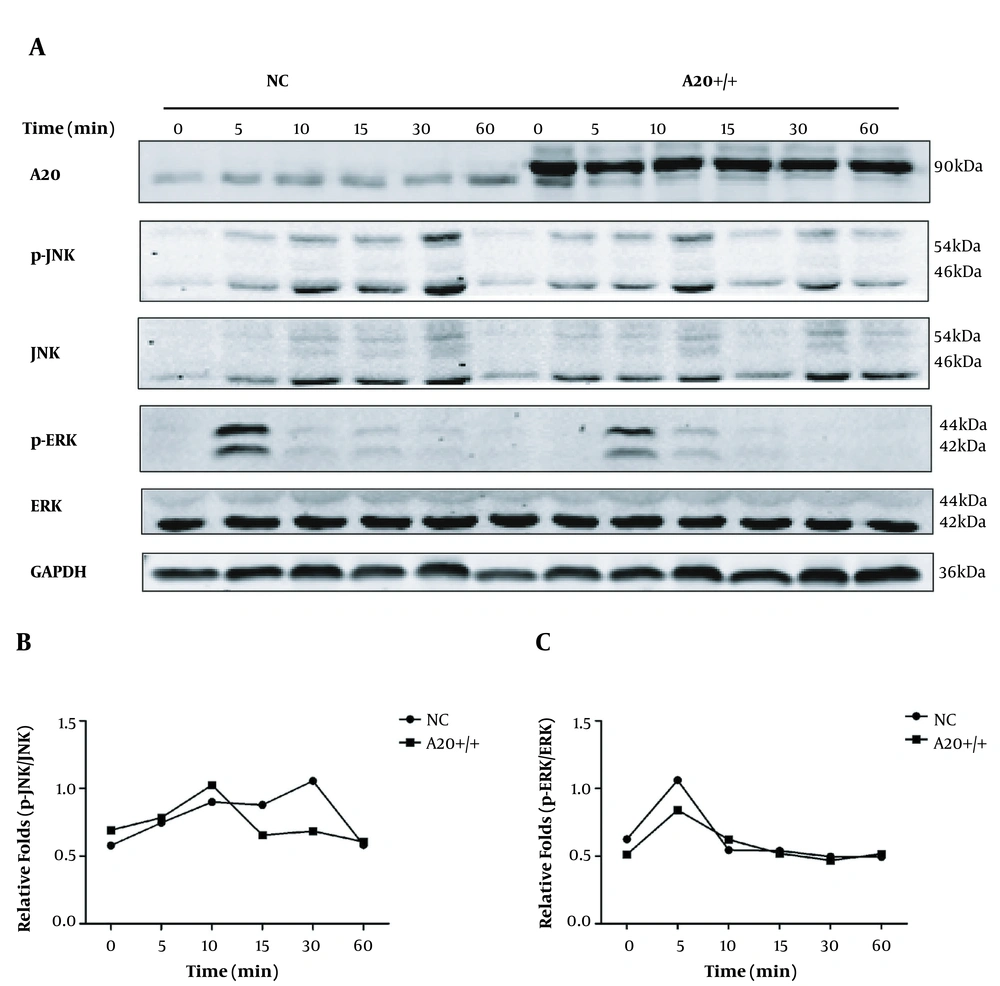
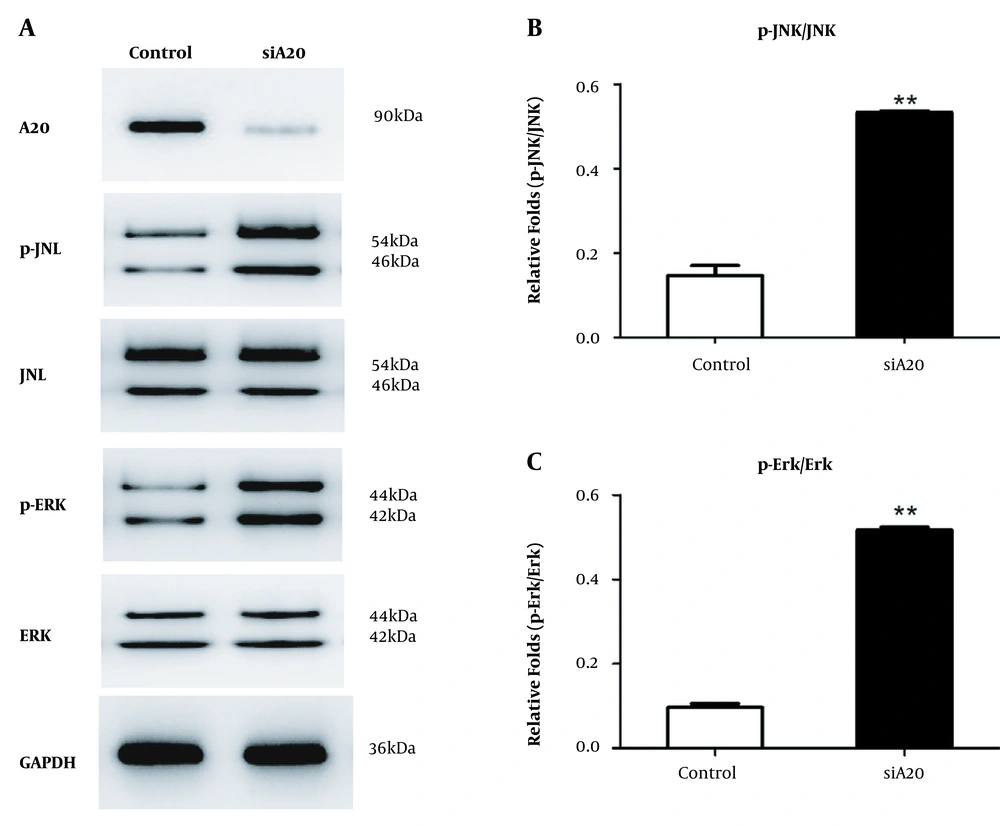
We confirmed that inflammatory cytokines expression was elevated by silenced A20 in HSCs stimulated by LPS. However, the underlying mechanism is unknown. Thus, we focused on MAPK/ERK/JNK signaling pathways that influence liver fibrogenesis (23-26). Our analysis showed that relative to controls, A20 overexpression in LX-2 cells stimulated with LPS for different durations significantly reduced phosphorylated ERK levels after 5 minutes, and phosphorylated JNK levels were also lower at 30 minutes relative to controls (Figure 3A-C). In the pre-experiment of A20 knockdown, we selected different durations and found that the band was most obvious at 30 minutes; thus, 30 minutes was chosen instead of the different durations according to A20 overexpression. A20 knockdown in LX-2 cells promotes phosphorylated JNK levels and phosphorylated ERK levels with LPS for 30 minutes (Figure 4A-C), suggesting that A20 modulates inflammation in HSCs via the MAPK/ERK/JNK pathway.
Function of A20 overexpression in MAPK/ERK/JNK pathway. A, Protein levels of phosphorylated MAPK/ERK/JNK in A20 overexpressing LX-2 cells exposed to 0.1 μg/mL LPS for indicated durations. GAPDH served as the loading control. B, Graph shows relative fold changes of p-JNK/JNK. C, Graph shows relative fold changes of p-ERK/ERK. P-JNK, JNK, p-ERK, and ERK represented as the sum of two bands.
Function of silencing A20 in MAPK/ERK/JNK pathway. A, Protein levels of phosphorylated MAPK/ERK/JNK in LX-2 cells transfected with A20-siRNA exposed to 0.1 μg/mL LPS for 30 min. GAPDH served as the loading control. B, Graph shows relative fold changes of p-JNK/JNK. C, Graph shows relative fold changes of p-ERK/ERK. P-JNK, JNK, p-ERK, and ERK represented as the sum of two bands.
5. Discussion
Here, we assessed the mechanism through which A20 mediates its anti-inflammation effects in LX-2 cells. The expression level of A20 is elevated in hepatic fibrosis tissue (17). Relative to controls, the fibrosis-related mRNA level of α-SMA, col-I, and col-III were increased in A20-siRNA LX-2 cells. Meanwhile, A20-siRNA cells significantly increased IL-6, TGF-β, and PDGF mRNA levels. Relative to controls, stimulating A20 overexpressing LX-2 cells with LPS for 5 and 30 minutes significantly reduced the levels of phosphorylated ERK and JNK, respectively. A20 knockdown in LX-2 cells promoted phosphorylated ERK and JNK levels with LPS for 30 minutes.
A20 influences various processes in the liver, including hepatic ischemia/reperfusion injury, liver protection, hepatocyte growth, hepatic inflammation, and apoptosis (10, 27, 28). Previous studies showed that A20 promotes hepatocytes proliferation and suppresses apoptosis in ALF rats (29). Liver A20 expression enhanced BALB/c mouse survival after hepatectomy (28). In A20 knockout mice, excessive liver inflammation and necrosis led to compensatory physiologic A20 upregulation in hepatocytes (30). Additionally, A20 confers proliferative advantage in hepatocytes and is a potential therapeutic target against liver injury after ischemia reperfusion (13). Thus, A20 has pro-proliferative, anti-inflammatory, and antiapoptotic effects in hepatocytes (27). We previously found that A20 may protect against liver fibrosis by downregulating inflammatory mediators in HSCs (17). A better understanding of A20’s role in HSCs may uncover novel therapeutic approaches for reducing liver fibrosis.
Hepatic stellate cells play a central role in the development of liver fibrosis via promoting ECM formation in hepatic tissues. Chronic liver inflammation alters HSCs from a quiescent state to activated state, myofibroblasts, and fibroblasts, via stimulation by cytokines, including IL-6, PDGF, and TGF-β (31, 32). IL-6 is a well-known pro-inflammatory cytokine, which can lead to liver injury and the occurrence of fibrosis (33). In the development of liver fibrosis, PDGF acts as a potent mitogen or activator of HSCs (34). TGF-β is one of the impotent growth factors associated with fibrosis progression in the liver (35). These cytokines induce the activation and proliferations of HSCs, which consequently result in liver fibrosis or cirrhosis (31-35). In our research, A20-siRNA cells significantly increased IL-6, TGF-β, and PDGF mRNA levels. Therefore, it is both interesting and important to know how A20 is linked to the above proinflammatory and profibrotic cytokines.
Previous studies (36) have shown that MAPK/ERK/JNK signaling controls various processes in HSCs, including cell growth, differentiation, survival, and apoptosis. ERK signaling has been shown to enhance liver fibrogenesis (23). A20 has been reported to downregulate TNFα-induced chemokine secretion in human colorectal cancer cells by suppressing ERK signaling (37). Moreover, inhibiting ERK and JNK phosphorylation in the MAPK pathway suppresses liver fibrosis (38). Some studies have found that A20 overexpression suppresses ERK and JNK activation, which are important for chemokine production (37, 39, 40). These findings suggest that A20 negatively modulates chemokine production by suppressing MAPK/ERK/JNK signaling, and A20 has potential as a therapeutic target against liver fibrosis. However, the possibility that A20 may become physiologically upregulated in HSCs in response to inflammation during liver fibrosis needs further investigation.
A20 protects HSCs against inflammation by inhibiting MAPK signaling, highlighting this pathway as a potential target for liver fibrosis treatment.