1. Background
Cytomegalovirus (CMV) is a double chain DNA virus that is a member of the Herpesviridae family and infects most people worldwide (1). Primary CMV infection can be asymptomatic or may occur as a self-limiting febrile disease in people with insufficient immunity. However, it may cause serious disease in susceptible individuals, such as solid organ transplant (SOT) recipients, in the form of a primary infection or as a result of the reactivation of a latent infection (1, 2).
CMV is an important cause of morbidity and mortality in SOT cases. Therefore, great efforts are made to prevent and manage CMV in SOT patients (2). In the United States, the overall CMV seroprevalence rate, which varies according to age, geography, and economic status, is reported to be 50% (3, 4). In Turkey, the overall seroprevalence of CMV-IgG is reported to be over 90% in all ages and genders (5, 6). In the literature, exposure to CMV has been reported to be 71.8% in liver transplant recipients according to preoperative tests (7).
Studies have reported that prophylaxis after transplantation significantly reduces the risk of CMV infection (8, 9). Without a prevention strategy, CMV infection and disease typically occurs within the first three months after SOT. It has been shown that if SOT patients do not receive prophylaxis until the 90th day after transplantation, 91.9% will have viremia, and 50-65% will develop symptomatic infection (8, 10).
2. Objectives
Prophylaxis application, effectiveness, postoperative CMV infection rates, and CMV treatment results vary between centers around the world. In this study, we aimed to contribute to the literature by sharing our CMV infection data in liver transplant recipients who underwent CMV prophylaxis in the Organ Transplantation Institute of Inonu University, Malatya, Turkey.
3. Methods
In this study, 1,090 patients who underwent liver transplant between January 2014 and December 2019 were retrospectively evaluated. All patients included in the study were over the age of 18 years. In accordance with the universal CMV prophylaxis protocol, valganciclovir (900 mg oral daily) prophylaxis was given to all patients in the first 100 days after transplantation. In the follow-up of liver transplant recipients, molecular screening tests for CMV was carried out in accordance with the recommendations of international guidelines. EZ1 virus Mini kit V2.0 (Qiagen, Germany) was used for CMV DNA extraction, and application was executed with CMV Qs-RGQ Kit (Qiagen, Germany) on Rotor Gene Q 5 Plex HMR (Qiagen, Germany). CMV DNA positive patients underwent weekly DNA monitoring until they received two consecutive negative reports. The standard immunosuppressive treatment consisted of a calcineurin inhibitors (CNIs), including tacrolimus or cyclosporine, and steroids. Mycophenolate mofetil, basiliximab, and everolimus were among the other immunosuppressive agents used. CMV infection and disease were defined in accordance with previously published definitions (2).
3.1. The Diagnosis of CMV Infection
After transplantation, CMV infection was diagnosed based on the replication of CMV-DNA (copy/mL) by quantitative nucleic acid amplification test (QNAT). The CMV-DNA threshold value was accepted as 1000 copies/mL in our center, and values above this threshold were evaluated in favor of CMV infection.
In patients diagnosed with a CMV infection after transplantation, the hospital database system was used to determine the number of CMV DNA copies from the postoperative samples on the day positivity is detected, as well as the preoperative CMV IgG results of the recipient and donor. Along with the recipient’s QNAT positivity, blood leukocyte, platelet, lymphocyte, and liver enzyme counts were recorded. Mortality was evaluated during the treatment period and one month after that. The results entered to the IBM SPSS statistics version 22.0 for Windows (SPSS, Inc., Chicago, IL).
3.2. The Diagnosis of CMV Disease
Patients were evaluated as probable CMV syndrome in the presence of at least two episodes of fever ≥ 38°C, new or increased malaise or fatigue, leukopenia or neutropenia, high level of hepatic aminotransferases, and the detection of CMV with QNAT in blood. For diagnosis of end-organ CMV disease, the results of histopathological biopsy were evaluated.
Intravenous ganciclovir was administered for the treatment. CMV antiviral therapy was maintained until QNAT was negative for at least 14 days. The duration of therapy was also recorded.
For the statistical analysis, categorical variables were compared with the Pearson Chi-Square test or Fisher’s exact test. A P < 0.05 value was considered significant.
The present study was approved by the non-interventional Ethical Committee of Medical Faculty at Inonu University, Turkey (approval no: 2020/262).
4. Results
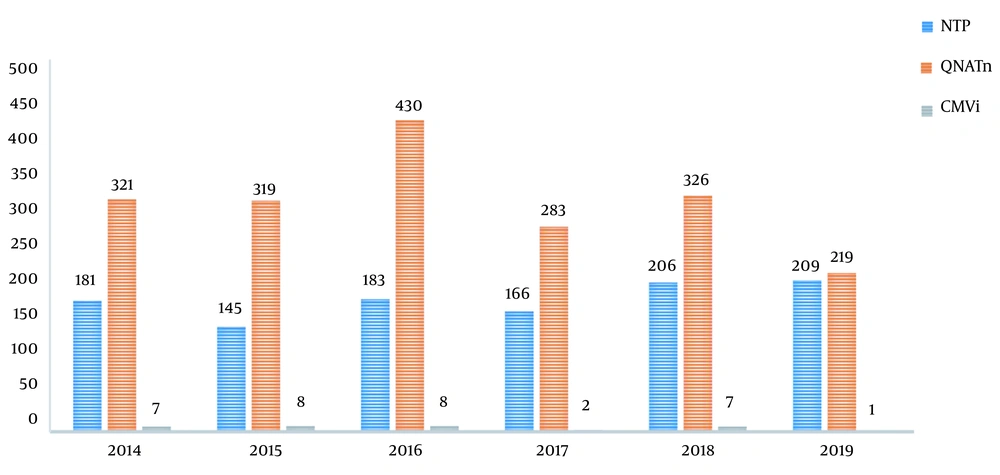
A total of 1,090 liver transplantations were performed in our transplantation institute between January 2014 and December 2019. The number of QNAT examined by year in cases of clinical suspicion or during CMV screening was reported as 321, 319, 430, 283, 326, and 219 from 2014 to 2019, respectively. A total of 1,898 tests were performed on 1,090 patients. Figure 1 displays the number of transplant cases per year, number of QNAT performed, and the number of patients with CMV infection. The clinical features and results of 33 (3%) patients whose CMV DNA in blood and/or other body materials was detected above 1000 copies/mL with QNAT were evaluated. The mean age ± SD of these 33 patients was 44.6 ± 14.9 years, and 21 (63.6%) were male. The demographic data and baseline recipient characteristics are shown in Table 1. Twenty-four of the donors were living, and the remaining nine were cadaveric. Eight out of 33 patients died during the antiviral treatment period or within one month of follow-up. Eight of the patients were determined to have end-organ CMV disease (Table 2), and 25 (2.3%) had probable CMV syndrome (Table 3). There was no patient with refractory CMV infection or with CMV replication who did not display the clinical signs and symptoms of the disease.
| Features | Values |
|---|---|
| Age, y | |
| Female | 39.3 ± 13.5 |
| Male | 47.6 ± 15.1 |
| Total | 44.6 ± 14.9 |
| Gender | |
| Female | 12 (36.4) |
| Male | 21 (63.6) |
| Donor Type | |
| Deceased | 9 (27) |
| Living | 24 (73) |
| Retransplantation | |
| Yes | 2 (6.1) |
| No | 31 (93.9) |
aValues are expressed as No. (%) or mean ± SD.
| Timing of Diagnosis (Days Post-Transplant) | Diagnosis/Classification | Method of Detection |
|---|---|---|
| 108 | Probable gastrointestinal CMV disease (2 patients) | Appearance of upper and/or lower GI symptoms and CMV demonstrated on colonoscopy material; but no macroscopic mucosal lesion |
| 34 | ||
| 15 | Probable gastrointestinal CMV disease (3 patients) | Appearance of upper and/or lower GI symptoms and CMV demonstrated on endoscopic material (bile); but no macroscopic mucosal lesion |
| 24 | ||
| 147 | ||
| 41 | Proven or definite CMV pneumonia (1 patient) | Pneumonia new infiltrates on imaging, hypoxia, tachypnea, and/or dyspnea combined with CMV documented in lung tissue by DNA hybridization techniques |
| 36 | Probable CMV pneumonia (1 patient) | New infiltrates on imaging combined with detection of CMV by quantitation of CMV DNA in BAL |
| 166 | Proven or definite CMV hepatitis (1 patient) | Deterioration in liver test results and CMV demonstrated with histopathology and documented by DNA hybridization techniques of liver tissue. |
| Case | Time of Diagnosis, Days Post-Transplant | CMV QNAT PCR, Copies/mL) | AST, U/L | ALT, U/L | Lymphocite,/mL |
|---|---|---|---|---|---|
| 1 | 20 | 9734 | 69 | 166 | 500 |
| 2 | 178 | 2500 | 14 | 27 | 500 |
| 3 | 111 | 1533 | 111 | 81 | 200 |
| 4 | 6 | 1820 | 6 | 9 | 1200 |
| 5 | 40 | 1650 | 40 | 24 | 530 |
| 6 | 31 | 13950 | 31 | 15 | 410 |
| 7 | 26 | 1647 | 62 | 55 | 100 |
| 8 | 115 | 1555 | 252 | 781 | 140 |
| 9 | 11 | 1331 | 66 | 85 | 170 |
| 10 | 41 | 7930 | 22 | 22 | 10 |
| 11 | 16 | 3050 | 60 | 60 | 104 |
| 12 | 14 | 14780 | 946 | 946 | 1328 |
| 13 | 22 | 2480 | 78 | 78 | 28 |
| 14 | 8 | 1536 | 58 | 58 | 79 |
| 15 | 175 | 2443977 | 60 | 60 | 118 |
| 16 | 43 | 2900 | 68 | 68 | 58 |
| 17 | 22 | 1307 | 92 | 92 | 50 |
| 18 | 131 | 22860 | 756 | 756 | 559 |
| 19 | 116 | 1452 | 47 | 47 | 62 |
| 20 | 90 | 97578 | 12 | 12 | 11 |
| 21 | 69 | 933 | 31 | 31 | 50 |
| 22 | 59 | 2208 | 11 | 11 | 11 |
| 23 | 7 | 4450 | 37 | 37 | 51 |
| 24 | 30 | 4360 | 3052 | 3052 | 496 |
| 25 | 7 | 79236 | 71 | 366 | 270 |
4.1. CMV QNAT Assessment
CMV QNAT was positive in the plasma of 26 patients, plasma and bile of three, plasma and colon tissue of two, and plasma and bronchoalveolar lavage (BAL) fluid of two patients. CMV QNAT values were determined as 1780 copy/mL and 2043 copy/mL in BAL fluid, 4450 copy/mL, 1390 copy/mL, and 1037 copy/mL in endoscopic material (bile). In the colonoscopic materials, CMV QNAT was detected as 194 copy/mL (simultaneous plasma was CMV QNAT 13841 copy) in one case and 401 copy/mL (simultaneous plasma was CMV QNAT 1702 copy/mL) in the other case.
The median level of plasma CMV QNAT was 2208 copy/mL (min 1033, max 2443977). The median day of detecting first positive CMV QNAT value was 41 (min 7, max 179) days. The median duration of treatment was 14 (min 1, max 37) days.
In 23 (2.1%) of the transplanted patients, positivity was detected in the early period within the first 100 days after transplantation. The other 10 (0.9%) patients were found positive for CMV QNAT after the prophylaxis period. However, all patients were diagnosed within the first six months after liver transplantation. Four of the 23 patients were diagnosed with CMV infection within the first 100 days, and four patients from the other 10 (diagnosed with CMV infection after 100 days) died (P = 0.174).
4.2. CMV Serostatus and Other Laboratory Findings
At the time of diagnosis, blood AST (U/L), ALT (U/L), white blood cell 103/uL), and lymphocyte count (/mL) values (mean ± SD) were detected to be 272 ± 609, 202 ± 316, 9227 ± 6476, and 570 ± 460, respectively. In patients with end-organ disease, mean liver enzymes were higher than those with CMV syndrome, while platelets were lower (Table 4).
| CMV Infection | Numbers | AST, U/L | ALT, U/L | WBC,/mL | Lymphosyt,/mL | Platelet, 103/mL |
|---|---|---|---|---|---|---|
| CMV syndrom | 25 | 222.3 ± 594.6 | 172 ± 294.9 | 9421.4 ± 6625.4 | 554.6 ± 464.6 | 102.3 ± 138.3 |
| End‐organ CMV disease | 8 | 553 ± 683 | 367.8 ± 414.1 | 8136 ± 6113.4 | 662 ± 477 | 72.6 ± 64.6 |
| Total | 272.4 ± 609.2 | 201.7 ± 316 | 9226.7 ± 6475.2 | 570.9 ± 460 | 97.8 ± 129.6 |
aValues are expressed as mean ± SD.
CMV serostatus of donors and recipients was not statistically different between diagnosis within the first 100 days and after after 100th day (P = 0.682) (Table 5).
| Early Diagnosis (Within the First 100 Days) (n) | Diagnosis After 100 Days (n) | |
|---|---|---|
| Preop CMVIgG positive (R+) (n) | 8 | 2 |
| Preop CMVIgG unknown (Ru) (n) | 15 | 8 |
Abbreviation: Preop, preoperative.
5. Discussion
Prophylaxis strategies for CMV infection are controversial in the world. Universal prophylaxis is commonly preferred in countries that have a high seroprevalence rate as it is more effective. However, the rate of late period CMV infections after prophylaxis is an important issue with changing data between centers (8, 10, 11). When CMV prophylaxis is not given in the postoperative period of SOT patients, majority of them develop a CMV infection. To prevent these infections, either prophylaxis or preemptive treatment protocols are conducted. Since CMV seropositivity was widespread, CMV prophylaxis and close follow-up with real-time polymerase chain reaction (PCR) was the standard procedure after liver transplantation. The main advantages of prophylaxis to preemptive therapy in the literature is that this approach is highly successful in preventing early CMV DNAemia/infection, and it can be applied relatively easily. Despite prophylaxis, in some patients were found early CMV DNAemia in this study. It may be thought an acute infection coincidence in the patients. The dosage of valganciclovir was passed from prophylaxis to therapeutic amount in this group. The lack of CMV serology control of recipient and donor candidates before transplantation was thought another possible reason. On the other hand, it has been reported that late CMV infection/disease is more common with prophylaxis. Despite prophylaxis, research has reported the rate of CMV disease in the D+/R+ group of liver recipients to be 5% (11). In our study, prophylaxis was applied, and the overall rate of CMV infection was 3% (n = 33). In the late period, 10 (0.9%) patients developed a CMV infection despite prophylaxis.
The use of common expressions in CMV infection and the definition of disease was discussed for the first time at the 1993 Fourth International CMV Conference in Paris (12). Later on, the importance of QNAT and histopathological tests for diagnosis and confirmation were emphasized. In that context, it has been emphasized to standardize the amount of CMV DNA (13). The World Health Organization (WHO) International Reference Standard for CMV quantification has become available to standardize values diagnostic of CMV infection (14, 15). Transplant centers are encouraged to achieve specific viral load thresholds based on the CMV QNAT test they use and the population at risk (2). In the study by Wadhawan et al., the viral load threshold value was specified as 500 copies/mL (10). Other studies reported that CMV viral loads above 1,000 copies/ml were generally associated with symptomatic CMV infections (16). In our center, CMV infection was determined at a CMV DNA cut-off value of 1000 copies/mL after transplantation.
CMV infection is characterized by the detection of CMV replication in patients regardless of symptoms. CMV replication can be detected by QNAT, antigen test, and culture. CMV disease is categorized as CMV syndrome and end-organ CMV disease (gastrointestinal disease, pneumonia, hepatitis, etc.) accompanying certain symptoms, including fever and/or weakness, and leukopenia or thrombocytopenia (17). In a single-center study on CMV syndrome and end-organ CMV disease, the number of CMV syndrome cases was 8 (2.4%), and the number of end-organ disease cases was 1 (0.3%) in 338 liver transplant patients over 5 years (10). In this study, 25 (2.3%) cases were diagnosed with CMV syndrome and 8 (0.7%) with end-organ CMV disease among 1,090 liver transplant patients over six years. CMV DNA was detected in the colon biopsy material of two cases with upper and lower gastrointestinal symptoms. It was also detected in the bile material taken by endoscopy in three cases exhibiting upper gastrointestinal symptoms. In these cases, QNAT also showed the presence of CMV DNA in blood. However, pathological confirmation could not be done in these cases, and they were characterized as having probable gastrointestinal CMV disease. The lack of histopathological documentation prevented the diagnosis of a proven gastrointestinal CMV disease.
In the literature, absolute lymphocyte count, which was shown to be associated with relapse in 33 of 170 participants (19.4%), was reported to be on average 1.08 ± 0.69 cells/µL in relapse-free patients and 0.73 ± 0.42 × 103 cells/µL in relapsed patients (18). In this study, the absolute lymphocyte count was 570.9 ± 460 cells/mL. However, no relapse was observed in the patients. On the other hand, absolute lymphocyte count remains both supportive and easily available in the diagnosis of CMV infection.
Diagnosis of CMV hepatitis requires histopathological and immunohistochemical (IHC) confirmation with elevated liver enzymes. There is no probable definition for CMV hepatitis (16). In only one patient, CMV hepatitis was diagnosed with definitive liver histopathological confirmation. The AST level of this patient was 241 (U/L), ALT was 312 (U/L), and CMV PCR was 1,310 copy/mL, and the diagnosis was confirmed on the postoperative 166th day. The duration of treatment was 29 days.
CMV QNAT in BAL fluid can be used to diagnose possible CMV pneumonia, and it is also recommended to define a viral load threshold (2). In our two patients who had CMV presence in BAL fluid, CMV QNAT values were determined to be 1,780 copy/mL and 2,043 copy/mL. One of the cases was characterized as histopathologically verified "proven pneumonia" and the other as “possible pneumonia”.
After antiviral prophylaxis, late-onset CMV disease associated with poor long‐term outcome can be seen. Patients may be monitored periodically using CMV QNAT for a certain time period even if they have completed antiviral prophylaxis. Virus quantification has been used as a method of direct measurement of the copied virus. Viral load assays play a significant role in patient management (19). The duration and interval of CMV monitoring following cessation of prophylaxis is not precisely established. One study reported that monitoring with two-week intervals was not clinically helpful in catching late-onset CMV disease after prophylaxis in SOT (17). In our study, a total of 1,898 tests were performed on 1,090 patients. Using QNAT, 33 (3%) patients had CMV DNA above 1000 copies/mL in blood and/or other body materials. Within the first 100 days after the operation, 23 (2.1%) of the transplanted patients were found to be positive for CMV DNA. The other 10 (0.9%) patients were found positive after the prophylaxis period was completed. Four of the 23 patients were diagnosed with CMV infection within the first 100 days, and four of the other 10 patients (diagnosed with CMV infection after 100 days) who had completed their prophylaxis died. There was no statistically difference in deaths between the two periods (P = 0.174). In this regard, we cannot conclude that they died due to CMV infection alone; hence, we need further prospective clinically controlled studies.
Another issue is duration of prophylaxis and duration of treatment. Protocols may vary according to centers. While some studies extended the treatment period up to three months, other studies proposed a longer period for prophylaxis (8, 20). We started prophylaxis in the first 10 days after surgery and it lasted for about three months (almost 100 days). The infection rate after prophylaxis (0.9%) was lower than the infection rate (2.1%) seen during prophylaxis. This suggested that it was not necessary to prolong prophylaxis time. The treatment period lasted until two CMV PCR results were negative for at least two weeks, with the mean ± SD being 18.48 ± 10.2 days. Since two cases died immediately after initiating treatment, their treatment could not be completed. Our longest treatment period was 37 days.
5.1. Conclusions
The main limitations of this study include the absence of a clinical control group, non-prospective nature of the study, and lack of CMV serostatus control. However, this study research a descriptive and self-assessment study. We consider close clinical follow-up as an important key point in liver transplant recipients who may face CMV infection. In addition, strictly monitoring CMV DNA by determining a certain cut-off can make the task easier.