1. Background
Hepatic fibrosis is a prevalent pathophysiologic process caused by chronic liver damage characterized by the excessive accumulation of extracellular matrix (ECM). Hepatitis B and hepatitis C virus (HBV and HCV), alcoholic steatohepatitis (ASH), non-alcoholic steatohepatitis (NASH), and genetic and autoimmune diseases are the foremost reasons for chronic liver disease (1). If the chronic liver disease continues and develops, it may lead to hepatic fibrosis, cirrhosis, hepatocarcinoma, and even liver failure (2). Hence, one of the new strategies in preventing the progression of liver fibrosis is to identify new anti-fibrotic agents. In the development of liver fibrosis, hepatic stellate cells (HSCs) activation is significant. These cells are located between hepatocytes and sinusoidal cells and are primarily responsible for producing abundant ECM in the liver (3). Hepatic stellate cells are activated by inflammatory factors such as Interlukin1β and Tumor necrosis factor-α (TNFα), growth factors including transforming growth factor beta (TGF-β), chemokines, and oxidative stress species (ROSs). Activated HSCs, through ECM remodeling, induce collagen deposition that leads to the development of liver fibrosis (4, 5).
NADPH oxidase (NOX) is an enzyme complex that produces ROSs from molecular oxygen. Among NOX activators, cytokine TGF-β has a major role due to its Smad3 transcription factor (6, 7). TGF-β activates the signaling pathway through its receptor. TGF-β binds to type II receptor (TβRII), and it phosphorylates, which in turn causes the phosphorylation of type I receptor (TβRI). The activation of TβRI leads to the phosphorylation of Smad2/3, which eventually binds to Smad4. Consequently, Smad heteromeric complex is formed and transported into the nucleus and activates the genes involved in fibrosis, such as NOXs and ECM proteins coding genes (8). Recent studies have shown that TGF-β signaling pathway blockers can inhibit fibrosis by inhibiting the activation of Smad2 and Smad3 with limited side effects in animal models. Thus, blocking the TGF-β/Smad3 signaling pathway is a common way to inhibit the activation of HSCs and reduce hepatic fibrosis (9, 10).
Quercetin, one of the predominant flavonoids, is detected in various vegetables and fruits, such as onion, kale, apple, and broccoli. Several useful properties of quercetin, including antioxidative, anti-inflammatory, antiviral, and anti-neoplastic effects, have been demonstrated in numerous in vitro and in vivo studies (11). Quercetin has been reported to decrease inflammatory genes and cytokine expression in macrophages. Treatment with quercetin dramatically suppressed NFκB activation in mice. Moreover, quercetin was found to attenuate the production of ROSs, mitochondrial dysfunction, and cell death (12, 13).
2. Objectives
Several studies have indicated the cytotoxic effect of quercetin in different cancer cell lines. Additionally, some studies have shown the effects of quercetin on the relative improvement of fibrosis in animals (14-16). However, the molecular mechanisms underlying the effects of quercetin on HSCs have not been completely determined. Therefore, in the current study, we investigated the effects of quercetin on the expression of NOX1, NOX2, and NOX4 and the phosphorylation of Smad3 in the presence and absence of TGF-β in LX2 as a human hepatic stellate cell line.
3. Methods
3.1. Cell Culture and Drug Treatment
LX-2 cells were kindly provided by Dr. S. L. Friedman (Mount Sinai School of Medicine, New York, NY, and USA). LX2 cells grown in DMEM were originally formulated with low glucose at 37°C in a humidified 5% CO2 atmosphere. Then, 2 ng/mL TGF-β was used to stimulate and activate LX2 cells, and the cells were kept in DMEM with 1% fetal bovine serum (FBS) for 24 h (17). Quercetin was purchased from Sigma-Aldrich (St. Louis, MO, USA). To prepare a quercetin stock solution, a certain amount of powder was weighed and dissolved in dimethyl sulfoxide (DMSO) according to the manufacturer's instructions. Then, different concentrations of quercetin (25, 50, 75, 100, and 125 μM) were treated with the TGF-β-activated HSCs. Then, the cells were collected and removed for real-time polymerase chain reaction (PCR) analysis. The present study was designed and conducted with the permission of the Ethics Committee of Jundishapur University Medical Sciences, Iran (IR.AJUMS.REC.1400.057).
3.2. MTT Assay
To investigate the cytotoxic effect of quercetin on the survival rate of LX2 cell line, in each well of a 96-well culture plate, about 5 ×103 LX2 cells were cultured and incubated for 24 h. The LX2 cell line was treated with different concentrations of quercetin (i.e., 25, 50, 75, 100, and 125 μM) (18) and incubated for 24 h. Next, cell culture media was exchanged with fresh media, and the MTT compound at a concentration of 0.5 mg/mL was added to each well for 4 h. After removing the MTT-containing media, 100 μL of DMSO was added to each well to dissolve the produced Formazan crystals. Finally, the absorbance of the samples was assayed at 570 nm.
3.3. RNA Extraction and qRTPCR
RNeasy kit (Qiagen Company, Germany) was used for extracting total RNA. The first-strand cDNA was synthesized from equal amounts of total RNA (1 μg) using SuperScript II reverse transcriptase kit with oligo dT (12-18) primer (Yekta Tajhiz, Iran), according to the manufacturer’s protocol. Real-time quantitative PCR of target gene was performed by RealQ Plus 2x Master Mix Green kit (Amplicon, Denmark). The primer pairs (forward/reverse) for cDNA amplification are listed in Table 1.
| Genes | Primers Sequences | Size of PCR Product (bp) |
|---|---|---|
| GAPDH | 237 | |
| Forward | 5′-TTCACCACCATGGAGAAGGC--3′ | |
| Reverse | 5′-GGCATGGACTGTGGTCATGA-3′ | |
| NOX1 | 192 | |
| Forward | 5′- CTGTTGCCTAGAAGGGCTCC-3′ | |
| Reverse | 5′- ACAGGCCAATGTTGACCCAA-3′ | |
| NOX2 | 119 | |
| Forward | 5′- CCTAAGATAGCGGTTGATGG-3′ | |
| Reverse | 5′- GACTTGAGAATGGATGCGAA-3′ | |
| NOX4 | 121 | |
| Forward | 5′-TGGAGGAAGAGGGAAGAGGT-3′ | |
| Reverse | 5′-AGAGCCAGATGAACCCAAGC-3′ |
Abbreviations: qRT-PCR, quantitative reverse transcription-polymerase chain reaction; NOX, NADPH Oxidase.
3.4. Immunoblot Analysis of phosphorylated Smad3C (p-Smad3C) in LX-2 Cell Line Activated with TGF-β1
LX2 cells were pretreated with the concentrations of 25, 50, 75, and 100 μM of quercetin for 30 min; then, the LX2 cells were treated with 2 ng/ml TGF-β for 30 min. Following that, cold phosphate-buffered saline (PBS) was used to wash the cells three times. LX2 cell lysates were prepared in radioimmunoprecipitation assay (RIPA) lysis buffer with protease and phosphatase inhibitors (Sigma Aldrich, USA). The bicinchoninic acid (BCA) method was used to quantify the total protein concentration. An equal amount of LX2 cell lysates (30 μg per lane) was run on 10% SDS-polyacrylamide gel electrophoresis (PAGE) under 130 volts for 90 min. The proteins were then transferred efficiently to the PVDF membrane (Millipore, USA) at a temperature of 4°C at a voltage of 100 V for 2 h (19). After transfer, the primary antibodies against p-Smad3C (1: 1000, Cell Signaling, USA) and GAPDH (1: 10000, Cell Signaling, USA) were incubated overnight at 4°C.
3.5. Statistical Analysis
Two-sample t-test (also known as the independent samples t-test) and one-way analysis of variance (ANOVA) were applied for experimental comparisons, using Prism 9 software (for Windows (GraphPad Software, USA).
4. Results
4.1. The Effect of Quercetin on the Survival of LX2 Cell Line
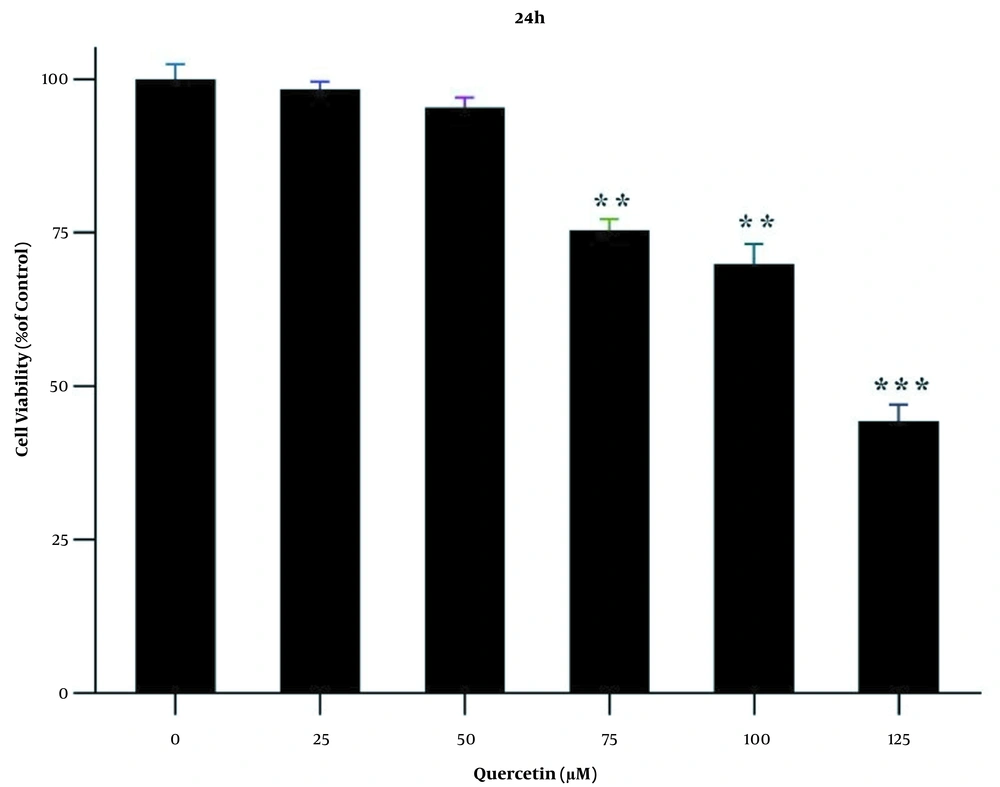
MTT test was performed at 24 h to achieve the appropriate concentrations of quercetin for drug treatment. For this purpose, first, LX2 cells were treated with different concentrations (i.e., 25, 50, 75, 100, and 125 μM) of quercetin for 24 h. At a concentration of 125 μM quercetin, the percentage of cell survival was significantly decreased compared to the control group (P < 0.001; Figure 1). For this reason, concentrations less than IC50 were used.
4.2. The mRNA Expression of NOX1, NOX2, and NOX4 in the Presence of TGF-β and Quercetin in LX2 Cell Line
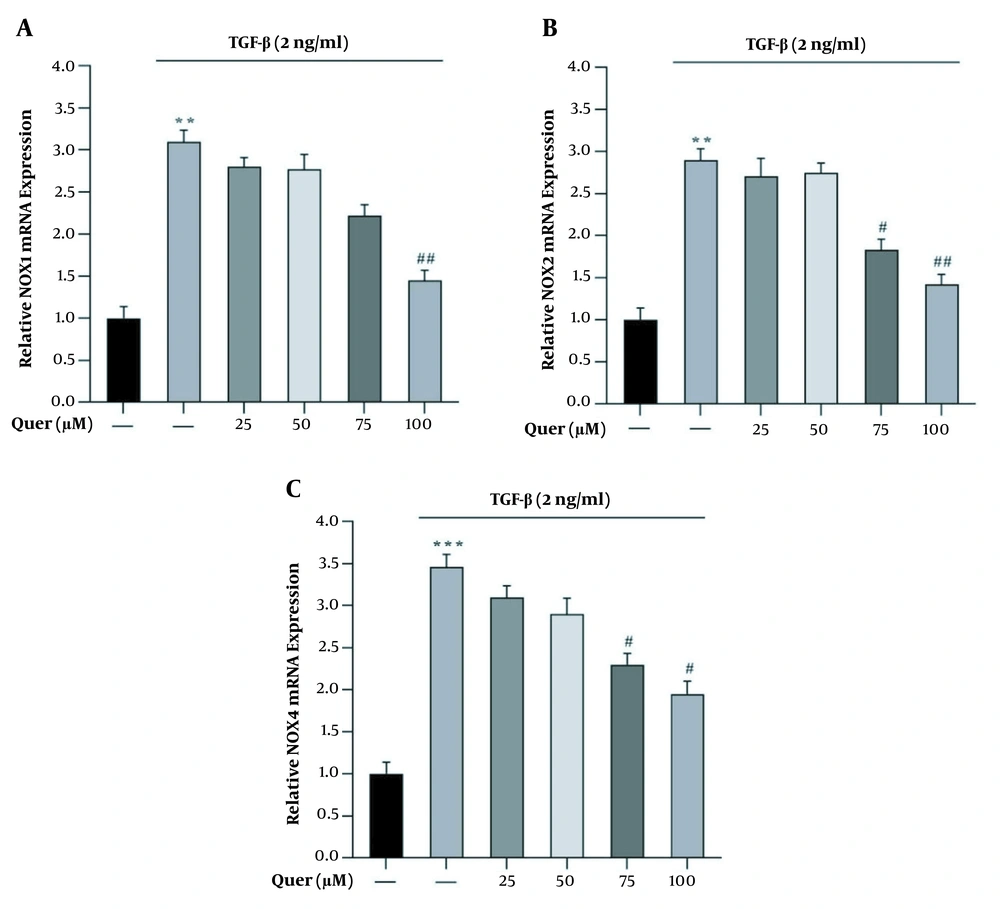
To evaluate the expression of NOX1, NOX2, and NOX4 genes, LX2 cells were treated with 2 ng/ml of TGF-β for 24 h. The results showed that the expression of NOX1, NOX2, and NOX4 genes in the presence of TGF-β was significantly increased compared to the normal control group. The cells were then treated for 24 h with different concentrations (i.e., 25, 50, 75, and 100 μM) of quercetin. Our results showed treatment with quercetin resulted in a significant and dose-dependent reduction in NOX1, NOX2, and NOX4 expression during 24 h compared to the TGF-β group (Figures 2A - C).
The effect of different concentrations of quercetin on the TGF-β-induced mRNA expression of NOX1, NOX2, and NOX4 in LX2 cells. A, NOX1; B, NOX2; and C, NOX4 mRNA expression induced by TGF-β with and without quercetin in LX2 cells. GAPDH was used as the reference gene (**P < 0.01, ***P < 0.001, #P < 0.05, ##P < 0.01). Data are represented as mean ± SEM (n = 3 biological replicates).
4.3. Effect of Quercetin on the Phosphorylation of Smad3C in LX2 Cell Line
Smads phosphorylation has a crucial impact on TGF-β-dependent transcriptional responses; thus, we assessed the effect of quercetin on Smad3C phosphorylation at Ser-423/425 in LX-2 cells. The cells were incubated with TGF-β1 and quercetin. Cell lysates were electrophoresed on SDS-PAGE and investigated with Smad3C phospho-specific antibodies for Ser-423/425. After induction with TGF-β1, p-Smad3C was significantly increased in the LX-2 cell line. Pretreated cells with quercetin caused a significant reduction in Smad3C phosphorylated at Ser-423/425 compared to that stimulated by TGF-β1 (Figure 3A and B).
Effect of quercetin pretreatment on the protein level of p-Smad3C induced by TGF-β. A, western blot for p-Smad3C protein expression induced by 2 ng/mL TGF-β1 with and without pretreatment with quercetin for 30 minutes; B, quantification of the relative p-Smad3C protein expression induced by 2ng/mL TGF-β1 with and without pretreatment with different concentrations of quercetin for 30 minutes. GAPDH was used as the reference gene [***P < 0.001, ##P < 0.01; data are represented as mean ± SEM (n = 3 biological replicates)].
5. Discussion
Liver fibrosis is a chronic disease due to a viral infection (including hepatitis C and B virus infections) and metabolic and genetic disorders, each of which can produce large amounts of ECM and promote fibrosis. Liver fibrosis is one of the main reasons for death in untreated chronic liver disease (20, 21). The lack of effective and efficient antifibrotic drugs for the treatment of liver fibrosis is a major global problem. Therefore, scientists are making great efforts to find safe and effective antifibrotic agents. In the current study, we demonstrated the cytotoxic effects of quercetin on the growth of LX-2 cells. Our results indicated that quercetin significantly inhibited the growth of LX2 cell line. Moreover, our results showed that quercetin reduced the expression of NOX1, NOX2, and NOX4 as profibrotic genes and the level of p-Smad3C in TGF-β-activated LX2 cell line.
Although several intracellular signaling pathways have been demonstrated to participate in the progression of liver fibrosis, TGFβ-activated pathways are one of the main goals of medical treatment (22, 23). In a progressive liver injury, TGFβ increases chronically, which in turn activates HSCs. When HSCs become activated, their phenotypes are changed and produce excessive ECM. Therefore, one of the beneficial ways for liver fibrosis treatment is preventing HSCs activation (24). Because TGF-β is an essential cytokine which implicates almost in all stages of liver physiology and pathology activities, its direct targeting may cause several unwanted controversial cellular responses (25). Thus, a compound that can modulate TGFβ-induced fibrogenic intracellular pathways has more medical implications.
There are increasing proofs indicating that flavonoids can inhibit and treat liver fibrosis via their antioxidant activity (26, 27). A study conducted by Yang et al. indicated that isorhamnetin, a natural flavonoid, could significantly inhibit TGF-β/Smad3 signaling and reduce oxidative stress, thus inhibiting HSC activation in CCL4-induced liver fibrosis (27). Kawada et al. found that quercetin could prevent the activation of HSC-T6 by reducing α-SMA expression and inhibit proliferation of these cells dose-dependently (26).
On the other hand, Wu et al. found that liver fibrosis produced by bile duct obstruction and CCl4-injection was constrained via preventing HSCs activation and decreasing autophagy (28). In our study, quercetin could significantly reduce the growth of LX2 cells and NOX1, NOX2, and NOX4 expression after 1 hour of incubation with TGFβ. The results of our study confirmed the findings of previous studies regarding the inhibitory effect of quercetin on the growth and proliferation of TGF-β-activated HSC. In the progression of hepatic fibrogenesis, activated HSCs play a critical role in the liver's primary source of ECM. Consequently, the excessive secretion of matrix proteins promotes hepatic fibrosis (29).
One of the hallmarks of chronic liver disease is oxidative stress, which affects the activation of HSCs. Reactive oxygen species produced by hepatocytes provide paracrine activation signals for HSCs. Emerging evidence shows that NADPH oxidases (NOXs) are a significant source of ROS. These enzymes play a vital role in the development of hepatic fibrosis (30, 31). Seven NOX isoforms are present in mammalian cells, all of which are integral membrane-bound enzymes with conserved structural properties that can produce superoxide and other ROS (32). Among them, NOX1, NOX2, and NOX4 are more expressed in HSCs and are involved in the development of liver fibrosis (33). In one study, NOX loss reduced liver damage from inflammation and fibrosis in CCl4- treated mice (34). NOXs are the main origin of ROS in the liver. NOXs mediate the fibrosis responses induced by TGF-β, PDGF, and angiotensin II in HSCs and macrophages. Zhan et al. (35) clearly showed that NOX activation and collagen production result from the phagocytosis of apoptotic bodies by HSCs after hepatocyte death. Another research has shown that the TGF-β-SMAD3 signaling pathway increases NOX1, NOX2, and NOX4 expression in HSCs associated with fibrosis (7).
TGF-β was known as a profibrogenic cytokine because of its role in activating HSC and the production of extracellular matrix (25). The main fibrogenic effect of TGF-β is usually mediated by the activation of HSC, the principal producer of extracellular matrix during liver fibrosis. Thus, TGF-β can be considered the starting point of liver fibrosis, and therefore, a very critical factor in the pathogenesis of liver fibrosis (6). To the best of our knowledge, our study is the first study regarding the effect of quercetin on the mRNA expression of NOXs in LX2 cell line. Our results indicated that quercetin significantly reduced the mRNA expression of NOX1, NOX2, and NOX4 and the protein level of p-Smad3C in TGF-β-activated HSCs. Our results suggested that quercetin can be considered as a potential antifibrotic compound in prospective studies.
5.1. Conclusions
Treatment with quercetin inhibits HSC activation in vitro. The mechanism proposed in this study is through the inhibition of the Smad3C signaling pathway. This is a key path in the synthesis and increase of ECM production. These data suggest that quercetin may be a potential compound for the treatment of liver fibrosis.
![Effect of quercetin pretreatment on the protein level of p-Smad3C induced by TGF-β. A, western blot for p-Smad3C protein expression induced by 2 ng/mL TGF-β1 with and without pretreatment with quercetin for 30 minutes; B, quantification of the relative p-Smad3C protein expression induced by 2ng/mL TGF-β1 with and without pretreatment with different concentrations of quercetin for 30 minutes. GAPDH was used as the reference gene [***P < 0.001, ##P < 0.01; data are represented as mean ± SEM (n = 3 biological replicates)]. Effect of quercetin pretreatment on the protein level of p-Smad3C induced by TGF-β. A, western blot for p-Smad3C protein expression induced by 2 ng/mL TGF-β1 with and without pretreatment with quercetin for 30 minutes; B, quantification of the relative p-Smad3C protein expression induced by 2ng/mL TGF-β1 with and without pretreatment with different concentrations of quercetin for 30 minutes. GAPDH was used as the reference gene [***P < 0.001, ##P < 0.01; data are represented as mean ± SEM (n = 3 biological replicates)].](https://services.brieflands.com/cdn/serve/3170b/8288e7320892409ad6dd101f9afe6f2b3dfec70f/hepatmon-116875-i003-F3-preview.webp)