1. Background
Coronavirus disease 2019 (COVID-19), due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), after being described for the first time in December 2019 in Wuhan, China, was announced pandemic by the World Health Organization (WHO) on 11 March 2020 (1). As of 14 March 2021, the world has reported 119 218 587 cases of COVID-19 with a total of 2 642 673 deaths (2). Recently, it has been documented that liver impairment affects 14% to 53% of SARS-CoV-2 positive cases over the course of the disease, and it seems to be more prevalent in severe COVID-19 cases than in mild ones (3). Furthermore, the prevalence of pre-existing chronic liver comorbidities is reported in about 3% of COVID-19 patients (4). Concerning the prevalence of viral hepatitis in the COVID-19-infected population, conflicting data are reported in China and the United States, probably reflecting different prevalence rates in the general population. A large case series of hospitalized patients from Wuhan, China, observed that 2.1% of patients with COVID-19 was infected with HBV, in contrast to 0.1% found in a similar analysis conducted in the Northeastern United States, while regarding HCV, this analysis reported a prevalence lower than 0.1% (5). Only a few reports from China reported higher morbidity, mortality, and a prolonged SARS-CoV-2 clearance in patients with HBV infection compared to those without HBV (6, 7). Therefore, a better understanding of SARS-CoV-2 infection when chronic viral hepatitis co-exists is very important.
2. Objectives
In this study, we aimed to determine the prevalence of pre-existing HBV and HCV infections in a population of SARS-CoV-2-infected subjects and evaluating whether this subgroup of patients experienced a more severe progression of COVID-19.
3. Methods
We retrospectively included all the subjects who have tested positive for SARS-CoV-2 on nasopharyngeal swabs performed from March to May 2020, regardless of the severity of COVID-19. Swabs could be performed either in the emergency room or after admission (due to suspected COVID-19) to hospital wards at S. Orsola Hospital in Bologna, Italy. We excluded all the subjects for whom HCV and HBV markers were not available at the time of SARS-CoV-2 detection. Among this population, we calculated the prevalence of patients with positive HBsAg, or with isolated HBcAb (i.e., HBsAg negative/HBcAb positive), or with a positive HCV antibody test. In addition, we determined the prevalence of patients with a chronic HBV- or HCV-related liver infection, defined as positive HBsAg and/or HCV RNA. Afterward, we created a matched cohort from the overall population to compare subjects with positive HBsAg and/or HCV RNA to those without chronic liver infections (i.e., with documented negative HBsAg and HCV RNA) in order to determine the rate of admission to the intensive care unit (ICU), usage of mechanical ventilation (MV) and occurrence of death, within the two groups. We also evaluated time for SARS-CoV-2 clearance (defined as at least two consecutive negative results for swabs collected 24 hours apart) in the two groups. Subjects with documented positive markers for HBV or HCV were matched to individuals with negative markers for HBV and HCV with respect to age, sex, and ward of swab execution. All patients positive for hepatitis were matched 1:5, except for two patients that were matched as follows: 1:2 and 1:3. Subjects with HIV infection or isolated HBcAb positivity were excluded from this analysis.
Patients’ characteristics were expressed as median (and IQR) or percentage when appropriate. We used logistic regressions to predict the risk for the established clinical outcomes in the two groups of patients. A P-value of < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS (version 24.0).
The study protocol was approved by the Ethical Committee of S. Orsola-Malpighi Hospital, Bologna, Italy. All procedures complied with the provisions of the Helsinki Declaration.
Exemption from the informed consent procedure was applied due to the emergency-care research setting.
4. Results
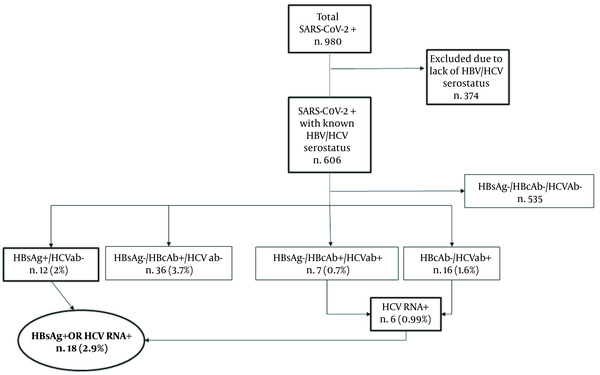
During the evaluation period, a total of 980 subjects positive for SARS-CoV-2 were considered. We included a total of 606 subjects who had concomitant available information about HBV and HCV serostatus. Among these, 12 (2%) were HBsAg positive, 43 (7.1%) were HBsAg negative/HBcAb positive, and 23 patients (3.8%) were positive for HCV antibody. We also observed 7 HBsAg-negative/HBcAb-positive subjects who had concomitant positive HCV serology. Six out of the 23 HCV-positive individuals had detectable HCV RNA. Overall, we found 18/606 (2.9%) subjects with a chronic HBV- or HCV-related infection (i.e. with either positive HBsAg or detectable HCV RNA), as depicted in Figure 1.
Afterward, we focused our attention on the subgroup of 17 subjects with chronic HBV and HCV infections and concomitant COVID-19, since a patient suffering from both chronic HCV infection and HIV was excluded from the analysis to reduce bias. The median age was 64 years, and 7 out of 17 subjects (41%) were male. No patient was receiving antiviral therapy at the time of our evaluation, and no one had a documented liver cirrhosis. Among 17 patients with documented HBV and HCV infections, 29% died, 29% were admitted to ICU, and 17.6% required MV support.
Then, as specified in the methods, we included 97 SARS-CoV-2-positive individuals in the matched analysis: 17 subjects with chronic HBV and HCV infections and 80 subjects with negative viral markers for HBV and HCV. Among this selected population, 48 cases (49.5%) were men with a mean age of 65 (IQR = 57-88) years. Sixteen patients (18 %) needed intensive care, and 10 cases (10%) needed MV. The mortality rate was 32% (31 individuals out of 97), with death occurring after a median of 7 (IQR = 2 - 17) days from COVID-19 diagnosis.
As presented in Table 1, no statistical differences were found in mortality rates in the two groups (P-value = 0.80, odds ratio (OR) = 0.87). Moreover, patients with HBV and HCV infections did not require MV more frequently compared to the other group (P-value = 0.27, OR = 2.38). Although it was not statistically significant (p value = 0.16, OR = 2.36), a higher rate of those positive for viral liver infections (rather than those with negative results for HBV/HCV) were admitted to the ICU (29% vs. 15%). Among these, 4 patients of those positive for viral liver infections had HBV, and 1 patient had HCV. Furthermore, we found a median time of virus clearance of 27.5 (IQR 20-38) days, with no difference between the two groups (P = 0.39).
| Matched Cohort (N = 97) | HBV/HCV-Infected (N = 17) | HBV/HCV-Uninfected (N = 80) | P-Value | |
|---|---|---|---|---|
| Age, y | 65 (57-88) | 64 (56-88) | 65 (57-88) | 0.91 |
| Male sex | 48 (49.5) | 7 (41.2) | 41 (51.3) | 0.81 |
| Death | 31 (32) | 5 (29) | 26 (32.5) | 0.80 |
| ICU admission | 17 (18) | 5 (29) | 12 (15) | 0.16 |
| MV | 10 (10) | 3 (17.6) | 7 (8.8) | 0.27 |
| SARS-CoV-2 clearance, d | 28(20 - 38) | 25 (17 - 37) | 28 (21 - 38) | 0.39 |
Demographic Characteristics and COVID-19-Related Outcomes in the Matched Cohort
5. Discussion
Liver damage in patients with COVID-19 has been widely described since the beginning of the SARS-CoV-2 pandemic, with up to 60% of infected patients presenting abnormal liver function (8). Many factors have been proposed for the development of hepatic injuries, such as immune-mediated damage, hypoxia, some medications, and viral hepatitis due to SARS-CoV-2 (3, 9). Reactivation of pre-existing liver disease has also been suggested as a causal factor: patients with chronic liver disease might have a higher risk for liver dysfunction during COVID-19, and subjects with chronic HBV-related infection undergoing specific therapies (such as tocilizumab or baricitinib) might experience HBV reactivation in the absence of antiviral prophylaxis (10). In a review entitled “COVID-19, MERS, and SARS with concomitant liver injury”, the authors stated that the impact of any pre-existing liver disease on SARS-CoV-2 infection course remains unclear and needs further investigations (11). A metanalysis by Mantovani et al. (4), including eleven observational studies involving patients with COVID-19, showed a relatively low prevalence of chronic liver disease at baseline, equal to 3%. HCV and HBV were reported as the main causes of liver diseases, but no specific data on the prevalence of these infections were provided. Similarly, we found a 3% prevalence rate of chronic liver disease associated with a documented underlying chronic HBV or HCV infections in our selected population. While the prevalence of positive anti-HCV antibodies among patients with COVID-19 has been described in the literature (12, 13), so far, no study has investigated the punctual prevalence of those with detectable HCV RNA and very few have evaluated the prevalence of patients with positive HBsAg among those diagnosed with COVID-19. For instance, in a cohort of 5700 patients admitted to hospitals for COVID-19 in the northeastern United States, HCV infection was observed in < 0.1% of patients (13), but information on HCV RNA level was not given. Chen et al. described a cohort of 123 patients with COVID-19 and HBV infection (6), however without mentioning HCV, presumably because of its low epidemiological impact in China. In our study, 23 cases (3.8%) presented a positive HCV serology, of whom 6 patients (0.99%) had detectable HCV viral load at the time of hospital admission for COVID-19 diagnosis, while 2% of all patients showed HBsAg-positive chronic infection. Recent reports about HBV and HCV infections among the overall population in Italy showed a prevalence of between 0.5% and 1% for HBsAg positivity and about 2.3% for HCV Ab positivity (14, 15), slightly lower than those recorded in our study sample.
In our study, patients with documented chronic HBV or HCV infection did not experience a more severe clinical course of COVID-19 compared to those with negative HBsAg or undetectable HCV RNA. As of today, infection with COVID-19 has been clearly associated with elevated mortality in patients with cirrhosis. A multicenter retrospective study on 50 patients with liver cirrhosis demonstrated a 30-day mortality rate of 34% (16). Our cohort did not include individuals with cirrhosis, which can partially explain our findings. Furthermore, differently from what was observed by Zha et al. (7), who measured a delayed SARS-CoV-2 clearance in patients with HBV, the median time of virus clearance was not affected by the presence of pre-existing HBV or HCV infection. The retrospective nature and the small sample size are the major limits of our study. Additionally, all the patients included in this study were recruited from a hospital setting representing a selection bias that has to be acknowledged.
In conclusion, chronic HBV or HCV infection (in the absence of liver cirrhosis) did not affect the prognosis of COVID-19 in our population. Further confirmations from larger and prospective studies with active screening for HBV and HCV, will be needed to provide clinicians with more specific information to manage COVID-19 in patients with HBV and HCV infections.