1. Background
Hepatitis B (HepB) infection is a major public health problem worldwide, which leads to chronic liver diseases such as liver cirrhosis and hepatocellular carcinoma. World Health Organization (WHO) estimated that more than two billion people are infected with hepatitis B virus (HBV) worldwide, of whom 350 - 400 million live with chronic infection, resulting in one million deaths each year (1). HBV infection is prevalent globally, with the highest endemicity in Southeast and Far East Asia and sub-Saharan Africa. In Mongolia, HBV prevalence is found to be 11.1% (2), which is much higher than in regions such as North America, Europe, and Japan. In addition, Mongolia has the highest incidence of liver failure and hepatocellular carcinoma mortality due to chronic hepatitis infection in the world (3).
Although antiviral treatments and management strategies are effective methods to control HBV infection, vaccination remains the most essential and economical option to reduce and potentially eradicate the prevalence of HBV infection (4). Following the global introduction of vaccines in 1982, Mongolia was one of 187 countries that integrated universal infant HepB vaccination into the national vaccination program in 1992, followed by the introduction of the mandatory pentavalent vaccine (combination vaccine against diphtheria, pertussis, tetanus, hepatitis B, and hemophilus influenza B) in 2005 (5, 6). Three doses of HepB vaccination are given 24 - 48 hours after birth, at two months of age, and at eight months of age (7). Since the introduction of HepB vaccination, the coverage rate has steadily increased and reached more than 90% in 2016 (8, 9). The mass vaccination of newborns has greatly prevented and controlled acute and chronic HBV infections in the younger Mongolian population while reducing asymptomatic carrier status (10). However, the high prevalence of hepatitis B surface antigen (HbsAg) (13 - 21.6%) among those born before the vaccination program and newly reported cases of infection among adolescents suggest that the virus is still a public health problem in Mongolia (11, 12).
Many countries, including Taiwan, Thailand, and China, have studied the long-term effects of HepB vaccination on immunity. Additional measures, such as the administration of a booster dose, are being considered, because the protective effect of the vaccine is shown to diminish over time (13, 14). For example, a previous study from the National Health and Nutrition Examination Survey in the United States reported that the serological evidence of immunity decreased from 56.8% to 44.4% within 6-8 years after HepB vaccination (15). In addition, after giving HepB vaccine at birth, the protective immune response was lost in 10% of the Taiwanese adolescent population (16). This may be associated with loss of immunologic memory, attenuation of anti-HBs, and increased risk of transmission (17). An anti-HBs titer of ≥ 10 mIU/mL is considered protective against HBV infection (18), and reduced vaccine immunity is of concern as it could lead to an outbreak of hepatitis infection and put an entire population at risk. Although several studies have been conducted in Mongolia, a nationwide study is needed to explore the general population's long-term immunity to HBV infection.
2. Objectives
We aimed to determine the prevalence of HBV infection in adolescents and young adults born since the introduction of the immunization program in 1991 and to investigate long-term immunity after HepB vaccination.
3. Methods
3.1. Study Design and Population
A nationwide population-based cross-sectional study was conducted between December 2016 and December 2018, recruiting 3591 individuals aged 10 to 27 years in Mongolia. A multistage randomized sampling method was used to select study participants. Mongolia is divided into four main geographic regions of West, Khangai, East, and Central, which in turn are subdivided into 21 provinces and the capital, Ulaanbaatar. Initially, several provinces from each region and districts from the city were randomly selected. Provinces are administratively divided into Soums, while districts are divided into Khoroos. In total, 13 Soums and Khoroos from the selected provinces and districts were randomly chosen. Finally, the sample from these areas was obtained using a list of registration numbers from the local health centers. Assuming 82.0% complete coverage with the HepB vaccine and 50.0% protection level after 5 - 15 years, the sample size was determined as 1144 using the following formula:
N: Population size (N = 903265); p: Expected proportion (p = 50.0%); r: Coverage rate (r = 0.819);
3.2. Data Collection
Data were collected by interviewing participants using a pretested questionnaire that included sociodemographic characteristics, medical history, and HBV vaccination status. Each participant’s vaccination history was evaluated by reviewing official vaccination records from the Immunization Department of the National Center of Communicable Diseases. Those who did not receive the full dose of HepB vaccination, were voluntarily vaccinated since the initial three doses, had hepatocellular carcinoma, were unable to participate in the study for medical reasons (i.e., could not provide a blood sample, were unable to answer questions, or were bedridden), or did not meet the inclusion criteria were excluded from the study. The inclusion criteria were as follows: age between 10 and 27 years at the time of the study; Mongolian citizenship; consent to participate in the study by themselves or their parents or guardians; and full childhood immunization coverage. Data collection was done after obtaining written informed consent from all participants and parents or guardians of children aged 10 - 15 years. The study protocol (22/1A) was subjected to scrutiny by the Ethical review committee of the Mongolian National University of Medical Sciences (MNUMS) (webpage of ethical approval code is: http://gp.mnums.edu.mn/?page_id=33).
3.3. Serological and Molecular Biology Tests
A 10 mL of whole blood was collected from participants in heparinized tubes and stored until serological analysis. Serum titers of quantitative hepatitis B surface antigen (qHBsAg), antibody to HBsAg (anti-HBs), and antibody to hepatitis B core antigen (anti-HBc) were determined by a two-step sandwich chemiluminescent enzyme immunoassay using a fully automated HISCL-5000 (Sysmex Corporation, Kobe, Japan), according to the manufacturer's protocol at the University Hospital of MNUMS. HBsAg presence indicates current hepatitis infection and is detectable within 12 weeks, while individuals who developed its antibody are considered non-infectious. Anti-HBc is present in both acute and chronic carrier states (19). The level of anti-HBs markers in the serum was assessed according to the WHO methodology and the standard set by the Minister of Health of Mongolia (Protocol number A/586) in 2019, according to Table 1. The anti-HBs level assessed immunity status: < 10 mIU/mL as negative, 10 - 100mIU/mL as weakly positive, and > 100 mIU/mL as positive. HBsAg negative, anti-HBs positive, and anti-HBc positive cases were considered immune due to natural infection, while HBsAg negative, anti-HBs positive, and anti-HBc negative cases were considered immune due to vaccination (Table 2).
| Interpretation | HBsAg | Anti-HBs | Anti-HBc |
|---|---|---|---|
| Susceptible to infection | - | - | - |
| Immune due to natural infection | - | + | + |
| Immune due to vaccination | - | + | - |
| Infected | + | - | + |
| More testing needed | - | - | + |
Interpretation of Hepatitis B Serologic Test Results
| Variables | Age (y), No. (col %) | |||
|---|---|---|---|---|
| 10 - 14 | 15 - 19 | 20 - 24 | 25 - 27 | |
| Gender | ||||
| Male | 466 (44.7) | 517 (44.6) | 313 (42.5) | 281 (43.0) |
| Female | 576 (55.3) | 642 (55.4) | 424 (57.5) | 372 (57.0) |
| Residential area | ||||
| Soum | 229 (22.0) | 194 (16.7) | 165 (22.4) | 145 (22.2) |
| Province center | 141 (13.5) | 121 (10.4) | 98 (13.3) | 87 (13.3) |
| Ulaanbaatar | 672 (64.5) | 844 (72.8) | 474 (64.3) | 421 (64.5) |
| HBV infection | ||||
| Positive | 41 (3.9) | 31 (2.7) | 47 (6.4) | 73 (11.2) |
| Negative | 1001 (96.1) | 1128 (97.3) | 690 (94.6) | 580 (88.8) |
| Total (row %) | 1042 (29.0) | 1159 (32.3) | 737 (20.5) | 653 (18.2) |
Demographic Characteristics and HBV Infection Status of the Survey Participants
3.4. Statistical Analysis
Statistical analysis was administered using the IBM SPSS Statistics 25.0 package (Chicago IL, USA). Quantitative data were presented in frequencies and percentages, and the chi-square test was used to detect differences among categorical variables. The age-specific geometric mean of anti-HBs was also estimated. Jonckheere-Terpstra test was used to detect significant trends between continuous variables. A P-value of ≤ 0.05 was considered statistically significant.
4. Results
A total of 3,591 individuals from Ulaanbaatar city and eight provinces of Mongolia participated in the study. Overall, 43.9% of the participants were male, 20.4% were from Soum, 12.4% were from the province center, and 67.1% were from Ulaanbaatar (Table 2). Four age groups were defined: 10 - 14 years (1042/29.0%), 15 - 19 years (1159/32.2%), 20 - 24 years (737/20.5%), and 25 - 27 (653/18.3%) years. All participants completed three doses of vaccine in childhood. Overall, 98.3% of the respondents reported being vaccinated as a child, and 57.2% answered they had been vaccinated within 24 hours of birth. Among adolescents and young adults aged 10 - 14, 15 - 19, 20 - 24, and 25 - 27 years, HBV infection was found in 3.9%, 2.7%, 6.4%, and 11.2%, respectively.
A total of 17.9% of participants had vaccine-induced immunity to HBV infection, 5.7% had infection-induced immunity, and 76.4% had insufficient immunoprotection and were HBV negative (Table 3). Immunity against HBV infection due to the vaccine was found in 22.1% of 10–14-year-olds, 16.5% of 15 - 19-year-olds, 15.7% of 20 - 24-year-olds, and 12.8% of 25 - 27-year-olds (P = 0.0001). The proportion of participants with vaccine-acquired immunity decreased with age, whereas naturally acquired immunity increased significantly. In addition, immunity status differed significantly between residential areas. Vaccine and infection-acquired immunity were the highest in rural Soum areas, while provincial centers had the highest number of individuals susceptible to HBV infection. There was no significant difference between anti-HBs serological levels and gender (P = 0.784).
| Variables | HBV Negative, Susceptible, No. (Row %) | Natural Infection-Induced, No. (Row %) | Vaccination Induced Immunity, No. (Row %) | P Value |
|---|---|---|---|---|
| Age (birth year) | < 0.0001 | |||
| 10 - 14 (2004 - 2008) | 479 (76.9) | 6 (1.0) | 138 (22.1) | |
| 15 - 19 (1999 - 2003) | 443 (81.1) | 13 (2.4) | 90 (16.5) | |
| 20 - 24 (1994 - 1998) | 254 (76.5) | 26 (7.8) | 52 (15.7) | |
| 25 - 27 (1991 - 1993) | 145 (63.9) | 53 (23.3) | 29 (12.8) | |
| Gender | 0.784 | |||
| Male | 584 (78.1) | 36 (4.8) | 128 (17.1) | |
| Female | 737 (75.2) | 62 (6.3) | 181 (18.5) | |
| Residential place | < 0.0001 | |||
| Soum | 269 (68.8) | 31 (7.9) | 91 (23.3) | |
| Province center | 176 (82.2) | 15 (7.0) | 23 (10.8) | |
| Ulaanbaatar | 876 (78.0) | 52 (4.6) | 195 (17.4) | |
| Total | 1321 (76.4) | 98 (5.7) | 309 (17.9) |
Characteristics of Participants Stratified by Post-vaccination Immunity Status
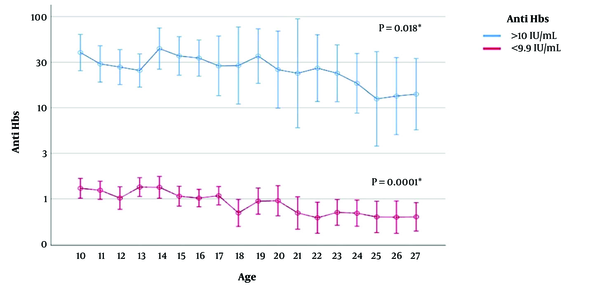
Figure 1 shows the geometric mean value of anti-HBs for vaccine-induced immunity of positive (> 10 mIU/mL) and negative (below 10 mIU/mL) participants by age. As the age of the participants increased, the geometric mean value of anti-HBs decreased significantly (P = 0.0001). In a vaccine-induced immune 10-year-old child, the geometric mean anti-HBs was 40.4 mIU/mL but 14.1 mIU/mL in a 27-year-old.
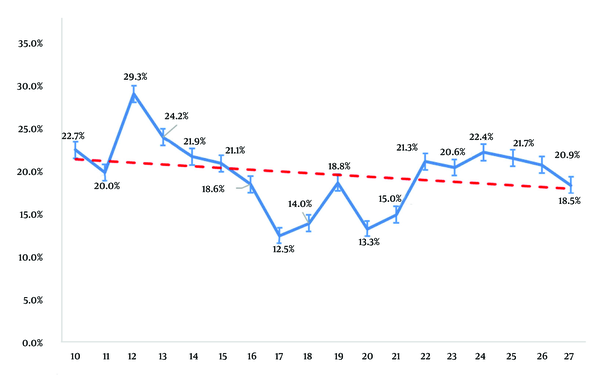
The vaccination-induced positive immunity rate decreased with age among HBV-negative (anti-HBs-positive, HBsAg-negative) individuals born after the introduction of the immunization program (Figure 2). Immunity rates were 22.7%, 20.0%, and 18.5% in 10-, 11-, and 27-year-olds, respectively (P = 0.0001). Anti-HBs titers reached their lowest level at the ages of 17 and 20 years.
5. Discussion
In Mongolia, every newborn has been vaccinated against hepatitis B infection for the last three decades. However, post-vaccination immunity has rarely been routinely tested in Mongolia. In our study, adolescents and young adults born after 1991 were selected from urban and rural areas, covering large geographic regions and the capital city. Vaccine-induced immunity to HBV infection was determined using anti-HBs levels, which revealed that a large proportion of the study participants had < 10 mIU/mL, or below the recommended protective level. A substantial number of participants were diagnosed with HBV infection, especially in the older age groups, indicating a high HBV disease burden in Mongolia. In addition, antibody titers steadily decreased with age, while infection-related immunity increased. Although women were in the majority in our study, there was no association between gender and HepB immune response, which was consistent with previous studies (17, 20).
In our study, vaccination-induced immunity against HBV infection was negative in 79.6%, weakly positive in 16.7%, and positive in 3.7%. Despite adequate HepB vaccination coverage rate, a consistent decrease in vaccine-associated immunity was observed as immunity in adolescents aged 15 - 19 years was low (16.5%), while the lowest value was seen in those aged 20 - 27 years (12.8%). The 10- to 14-year-old-group had the strongest vaccine-induced immunity, and the likelihood of seroprotection was 3.3 times higher among those vaccinated in the birth cohort 2004 - 2008 than among those born between 1991 and 1993. The results indicate that the prevalence of HBV infection was reduced in the younger population compared to the pre-vaccination period; however, the proportion was still higher than other countries with a national immunization program (21).
Our findings revealed that the prevalence of HBV infection increased with age, with the lowest prevalence in the 10-14 age group (3.9%) and the highest in the 25 - 27 age group (11.2%). The seroprevalence of HBV in the adult population of the pre-vaccination period was estimated to be 11.8% in rural and urban areas of Mongolia (22, 23). Several studies have been conducted in Mongolia on HepB vaccination, immunity, and infection. The incidence of HBV infection was twice as high in the pre-vaccination period compared to our study findings. The national survey by Davaalkham et al. included 1145 children aged 7 - 12 years born from 1992 to 1997, and the immunization coverage for HepB infection was 60.1% (12). The prevalence of HBV infection in children born after the universal immunization program was 15.6% (95% confidence interval (CI): 13.4 - 17.8%), and the proportion of HBsAg carriers was 5.2% (95% CI: 3.9 - 6.5%), which was higher than in other countries with a national immunization program. Moreover, the prevalence of HBV infection was significantly higher in children living in rural areas and was associated with older age and male sex (24). In our study, Soum residents had a relatively high prevalence of naturally induced immunity, which could be explained by the rate of infection in these areas. People living in urban areas tend to have better access to vaccines, which can be used to explain higher immunity due to vaccination. In contrast, residents of provincial centers were most susceptible to HBV infection, possibly because of limited supply and loss of vaccine efficacy during transport or storage. A meta-analysis by Nimadava et al., which included eight studies with more than 17,000 participants, reported that the proportion of HBsAg carriers in a relatively healthy unvaccinated population was 11.8 ± 1.8%, whereas the prevalence in a vaccinated population was 3.6 ± 1.5% (25). A more recent meta-analysis from the WHO, which included 26 studies that mostly were from China, found that the prevalence of HBV infection in an unvaccinated population was 3.5 - 16.5%, while it was 0.3 - 8.5% in a vaccinated population (26). In a study of children under two years of age by Edstam et al., 81.3% of participants developed vaccination-induced immunity. They reported that the mean anti-HBs concentration was 93.7 ± 21.8 mIU/mL, while vaccination-induced immunity in children aged one to three years in Ulaanbaatar city was 41.1% previously (5). The difference in the results may be due to the fact that the above studies recruited children under five years of age, and immunity was established 1 - 3 years soon after vaccination.
Several other studies found consistent results with our findings, such that the geometric mean value of the anti-HBs decreases with age. For instance, infants born in Taiwan were vaccinated immediately after birth and followed up. Anti-HBs antibody titers were 98%, 93%, 84%, and 66.0% at 2, 4, 5, and 10 years of age, respectively, and found that initial high antibody titer was associated with less risk of antibody decrease (27). Similarly, in a study of 5,024 children aged 5 - 11 years in India, anti-HBs antibody titers decreased with age, and no gender differences were observed (28). Moreover, in a study of 44 individuals vaccinated 32 years before in Alaska, 51% had anti-HBs levels below 10 mIU/mL (29), while in a 20-year follow-up study in Thailand, the percentage of anti-HBs levels of 10 mIU/mL or higher decreased from 100% one year after vaccination to 64.0% after 20 years. On the other hand, the geometric mean value of anti-HBs decreased from 4436.3 mIU/mL one year after vaccination to 20.4 mIU/mL after 20 years (13). Similar findings were observed in our study; namely, the proportion of participants who had serum anti-HBs greater than 10 mIU/mL decreased with age, and the average vaccination-induced immunity level was 25.9 mIU/mL.
Receiving three complete doses of HepB vaccination results in a minimally acceptable immune response. However, anti-HBs titer tends to decline sharply within the first year and slowly thereafter (30). The initial antibody concentration after the first vaccination, the number of vaccinations and dosage, age at immunization, and genetic factors all play a role in determining individual immunity and the duration of its protective effect (31). Moreover, single-point mutations have been associated with diminished vaccine efficacy. For example, a predominant G145R mutation that neutralizes immunogenic activity was more prevalent in the post-vaccination period than in the pre-vaccination period, particularly in vaccinated children (32).
Although guideline published by the WHO for vaccination against the HBV infection does not recommend booster vaccination in immunocompetent individuals who have received a full dose and responded well (33), booster vaccination is generally advised when anti-HBs serum levels are low (14), particularly in age groups engaged in activities with a high risk of transmission and in health care workers. In addition, people who are immunocompromised (e.g., due to chronic renal failure or HIV/AIDS), or undergo hemodialysis are recommended to receive a booster dose when anti-HBs levels are below 10 mIU/mL as they are predisposed to slower immune response (34). However, previous studies suggest that reduced or even undetectable anti-HBs serum levels do not necessarily indicate a loss of humoral immune memory to HBV infection (29, 35). In fact, individuals vaccinated during childhood have been shown to demonstrate immune memory to HBV at the age of 24 years (36, 37). Moreover, it has been recently reported that an anti-HBs level of ≥ 2 mIU/mL is sufficient to elicit an immune response following a HepB booster (38, 39). In addition, it has been reported that 97.9% of adolescents who were vaccinated in infancy and received a single booster dose against HBV infection developed seroprotection when tested one month later (40). Pretesting vaccine-induced and acquired immunity prior to booster vaccination and administering only to individuals with an anti-HBs level < 10 mIU/mL can be cost-effective in developing countries.
5.1. Conclusions
Since the introduction of the national hepatitis B immunization program in Mongolia in 1991, a small proportion of the population had intact immune memory. Immunity to HBV infection and geometric mean concentration of anti-HBs after primary vaccination decreased in an age-dependent manner while HBsAg seroprevalence increased. The findings implicate the need to implement an HBV infection booster vaccination program or long-term HBsAg monitoring to detect HBV infection carrier status in adolescents and young adults. Further studies are needed to investigate breakthrough infections and causes of reduced efficacy in vaccinated individuals, as well as the prevalence of HBV infection in the unvaccinated population in Mongolia.