1. Background
The global epidemic of hepatitis B virus (HBV) infection is the leading cause of cirrhosis and liver cancer, which seriously affects people’s health (1). Although global medical technology has made remarkable progress in recent years, HBV-related liver diseases, especially liver cirrhosis and hepatocellular carcinoma (HCC), are still challenging. It was reported that cirrhosis and liver cancer due to persistent HBV infection accounted for approximately 709,400 deaths annually in the world (2). One of the reasons is the lack of typical clinical symptoms and effective monitoring methods, so most patients are diagnosed in the advanced stage of the disease, which usually restricts the efficacy of therapies. Notably, monitoring the disease progression of HBV infection has been a main clinical issue. Biomarkers simultaneously reflecting liver injury, fibrosis, and carcinogenesis may be more conducive to predicting the progression of HBV-related liver disease and providing a basis for early clinical diagnosis and treatment.
Fibroblast growth factor 19 (FGF19, mouse homolog FGF15) is an atypical FGF mainly produced by the ileum (3). Due to the lack of a heparin-binding domain, FGF19 has a weak affinity for heparin sulfate (HS) and is effortless to spread from the secretory site to the blood as a hormone to regulate bile acid homeostasis, metabolism of carbohydrates, protein synthesis, lipid metabolism, etc. (3, 4). Previous studies found that FGF19 was associated with several types of liver diseases. For example, Brandl et al. (5) found that serum FGF19 enormously increased in patients with alcoholic hepatitis, and there was a significant negative correlation between FGF19 with the liver fibrosis stage as well as the level of polymorphonuclear cell infiltration. Other studies showed that liver expression of FGF19 was enhanced in primary biliary cirrhosis and correlated with the severity of the disease (6). But some researchers reported that serum FGF19 levels were inversely associated with hepatic damage in children with nonalcoholic fatty liver disease (NAFLD) (7). Furthermore, serum FGF19 was significantly increased in patients with primary biliary cholangitis-autoimmune hepatitis overlap syndrome (PBC-AIH OS), and modulation of serum FGF19 may provide a promising targeted therapy for patients with PBC-AIH OS (8). Luo et al. (9) revealed that the non-tumorigenic FGF19 variant effectively prevented and treated cholestasis liver disease and other diseases associated with bile acid dysregulation. Moreover, some studies demonstrated that FGF19 was a crucial oncogenic driver gene in HCC and correlated with poor prognosis (10-13). In addition, Wu et al. (14) pointed out that small proteins of hepatitis B virus surface antigen (SHBs) could activate the FGF19/JAK2/STAT3 signaling pathway through endoplasmic reticulum stress and induce epithelial-mesenchymal transformation process in HCC cells, thereby significantly increasing migration and invasion capacity of HCC cells. However, the value of serum FGF19 in HBV-related liver disease remains unclear.
2. Objectives
This study aimed to explore the clinical significance of serum FGF19 as a new biomarker for HBV-related liver disease.
3. Methods
3.1. Patients
A retrospective study included 37 patients with chronic hepatitis B (CHB), 33 patients with HBV-related cirrhosis (HBV-cirrhosis), and 32 patients with HBV-related HCC (HBV-HCC) admitted to Chongqing University Three Gorges Hospital from June 2019 to November 2021 were recruited for the study group. Inclusion criteria included (1) age is between 18 and 75 years with no gender limitation; (2) CHB is defined as patients with positive HBsAg for at least six months or HBV DNA > 2,000 IU/mL, which also requires serological or histopathological evidence, excluding cirrhosis and liver tumors; (3) HBV-cirrhosis is defined as HBV-infected patients with liver cirrhosis identified by imageology or histological diagnosis; (4) HBV-HCC is defined as HBV-infected patients with space-occupying lesions in the liver discovered by histopathological evidence or more than two clinical indicators, such as alpha-fetoprotein (AFP), ultrasound, and CT. Exclusion criteria included (1) autoimmune liver disease (ANA > 1/320), alcoholic hepatitis, drug, and toxic liver injury, and other conditions that can cause severe liver damage; (2) hepatitis, cirrhosis, and liver cancer are caused by other etiologies; (3) acute cardio-cerebrovascular accident and extrahepatic end-stage diseases; (4) pregnant, parturient, or lactating women; (5) other conditions are considered unsuitable for inclusion by researchers. In addition, 33 healthy people were randomly selected as the control group. Clinical data of each group were collected. The model for end-stage liver disease sodium (MELD-Na) (15) score was calculated from all patients with HBV-related liver disease in whom total bilirubin level (TBIL), international normalized ratio (INR), creatinine, and sodium levels were available. The Child-Pugh classification was calculated from all patients with cirrhosis in the study group based on the five indexes of TBIL, albumin (ALB), prothrombin time (PT), ascites, and hepatic encephalopathy (16). Within each index, a score of one to three is given depending on the severity of the abnormality. The final ordinal score then allows further classification into one of three Child-Pugh classes: Child-Pugh A (score 5 - 6), Child-Pugh B (score 7 - 9), and Child-Pugh C (score 10 - 15) (16). In this study, 61 HBV-infected patients had cirrhosis (33 patients in the HBV-cirrhosis group and 27 patients in the HBV-HCC group), which were divided into three grades according to the Child-Pugh classification, including 21 patients in Child-Pugh A, 23 patients in Child-Pugh B, and 17 patients in Child-Pugh C. The privacy rights of human subjects always were observed. This study was conducted in accordance with the Helsinki Declaration and was approved by the Ethics Committee of Chongqing University Three Gorges Hospital (dated: 28/01/2022, issue no: No.13, 2022).
3.2. Measurement of Serum FGF19
The serum specimens were obtained from patients after at least 8 - 10 hours of fasting. Moreover, all serums were stored at -80°C before laboratory testing. Concentrations of FGF19 in serum were quantified using ELIZA kits (R&D systems, catalog number DF1900), and Spectra Max M4 was used for detection. The experiment was carried out in strict accordance with the standard procedures of the reagent instructions.
3.3. Statistical Analysis
All data were analyzed using SPSS software, version 26 for Windows. Counting data were expressed as frequency, and chi-square test was used to compare the groups. Normality was determined with Kolmogorov-Smirnov test. Normally distributed data were expressed as mean ± standard deviation, while non-normally distributed data were expressed as median with interquartile range. Student’s unpaired t-test and ANOVA analysis were used to compare the difference between groups when the data satisfied normality and homogeneity of variance; otherwise, non-parametric tests were used. The Spearman rank correlation, Pearson’s correlation, or point biserial correlation method was selected for correlation analysis of data sets according to the characteristics of variables. The accuracy of HCC prediction by serum FGF19 was evaluated using the area under the receiver operating characteristic (ROC) curve. The area under the curve (AUC) was presented with a 95% confidence interval (CI). All analyses were two-sided, and P-values < 0.05 were considered statistically significant.
4. Results
4.1. Clinical and Laboratory Characteristics of Healthy Controls and Patients with HBV-Related Liver Disease
Patients in the HBV-cirrhosis and HBV-HCC group had significantly higher biomarkers of liver injury than CHB and control subjects (Table 1).
| Variables | Control (N = 33) | CHB (N = 37) | HBV-Cirrhosis (N = 33) | HBV-HCC (N = 32) |
|---|---|---|---|---|
| Age (y) | 57 (56, 62) | 45 (34, 50.5) a | 55 (50, 63.5) b | 56.5 (48.8, 67.5) b |
| Gender (M/F) | 23/10 | 17/20 | 20/13 | 25/7 |
| ALB (g/L) | 44.8 ± 3.1 | 45.8 ± 2.6 | 33.9 ± 7.7 a, b | 33.7 ± 7.5 a, b |
| TBIL (mg/dL) | 1.2 (0.8, 1.8) | 1.2 (0.9, 1.7) | 3.5 (2.1, 9.4) a, b | 3.3 (2.0, 10.3) a, b |
| ALT (U/L) | 15.1 (12.3, 25.5) | 22.0 (16.5, 36.6) | 35.0 (23.2, 51.3) a | 37.2 (24.2, 55.2) a, b |
| AST (U/L) | 19.3 (15.8, 21.5) | 24.0 (20.0, 31.4) | 48.4 (34.0, 81.9) a, b | 72.5 (27.0, 129.9) a, b |
| GGT (U/L) | 79.0 (64.5, 95.5) | 15.0 (12.0, 23.0) a | 40.0 (21.5, 74.5) b | 111.5 (54.0, 244.3) b |
| ALP (U/L) | 22.0 (15.5, 33.0) | 70.0 (55.0, 85.0) a | 99.0 (80.0, 147.0) a, b | 130.0 (91.0, 231.3) a, b |
| TBA (mg/dL) | 0.3 (0.2, 0.7) | 0.6 (0.2, 1.1) | 7.1 (2.7, 14.4) a, b | 3.3 (2.0, 15.0) a, b |
| HA (μg/L) | / | 66.2 (55.4, 87.1) | 388.3 (161.0, 655.3) b | 218.8 (114.9, 438.9) b |
| CIV (μg/L) | / | 17.9 (16.4, 19.9) | 81.1 (65.4, 146.2) b | 65.9 (43.4, 240.5) b |
| PIIINP (μg/L) | / | 21.9 (19.6, 25.6) | 47.5 (35.8, 125.0) b | 52.4 (32.4, 538.8) b |
| LN (μg/L) | / | 16.2 (12.4, 20.0) | 55.9 (28.9, 170.9) b | 60.4 (12.4, 186.4) b |
| CG (μg/L) | / | 1.5 (1.2, 2.6) | 16.6 (11.5, 30.0) b | 11.7 (4.5, 40.0) b |
| AFP (ng/mL) | 3.2 (2.6, 4.8) | 2.9 (2.0, 3.9) | 4.7 (2.1, 9.2) | 226.3 (29.2, 1200) a, b |
| LgHBV-DNA (IU/mL) | / | 4.1 (2.8, 5.4) | 2.3 (2.3, 4.5) b | 3.1 (2.3, 4.6) b |
Clinical and Laboratory Characteristics of Healthy Control and Patients with HBV-Related Liver Disease
4.2. Comparison of Serum FGF19 Levels in the Four Groups
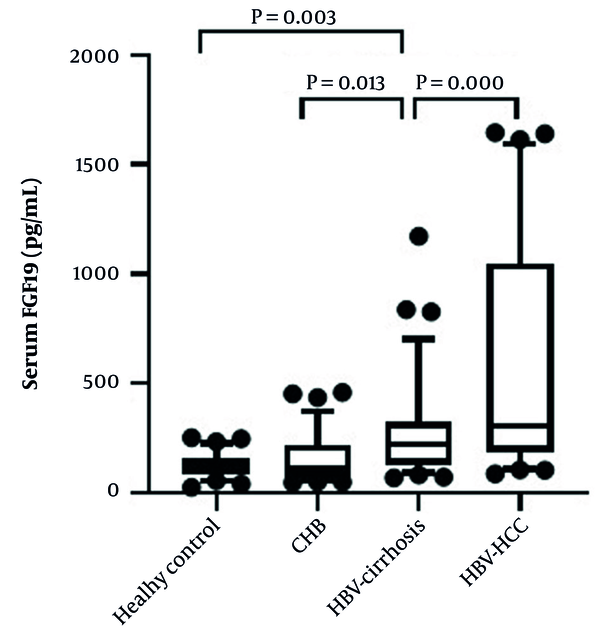
The expression of serum FGF19 in the HBV-related liver disease group was significantly higher than the control subject [114.2 (85.6, 157.3) pg/mL]. Furthermore, serum FGF19 levels increased sequentially among CHB group [112.2 (70.6, 221.5) pg/mL], HBV-cirrhosis group [222.5 (127.1, 327.3) pg/mL], and HBV-HCC group [307.5 (186.0, 1047.9) pg/mL]. However, there was no significant difference in serum FGF19 levels between the healthy control group and the CHB group (Figure 1).
Comparison of serum FGF19 levels in the four groups. Data are shown as box-and-whisker plots. The horizontal line in the middle of each box indicates the median value. The top and bottom borders of the boxes represent the 75th and 25th percentiles, respectively; the whisker represents the 10th and 90th percentiles, respectively; and the dots represent the outliers.
4.3. The Relationship Between Serum FGF19 with Routine Clinical Indicators in 102 Patients with HBV-Related Liver Disease
4.3.1. Serum FGF19 Levels Were Positively Associated with Serum Markers of Cholestasis and Hepatocyte Injury in HBV-Related Liver Disease
Serum levels of TBIL, direct bilirubin (DBIL), gamma-glutamyl-transferase (GGT), alkaline phosphatase (ALP), and total bile acid (TBA) are typical indicators to reveal cholestasis. Notably, it was a significant positive correlation between TBIL and FGF19, DBIL and FGF19, GGT and FGF19, ALP and FGF19, as well as TBA and FGF19 in the serum of patients with HBV-related liver disease in this study. In addition, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are mainly used to monitor the extent of hepatocellular damage. Our study found that serum FGF19 levels in patients with HBV-related liver disease also had a significantly positive correlation with ALT and AST. Furthermore, the correlation between FGF19 and AST was more robust than that with ALT (Table 2).
| Feature | Serum FGF19 | |
|---|---|---|
| Correlation Coefficient | P-Value | |
| Age | 0.305 | 0.002 |
| Gender | -0.019 | 0.853 |
| AFP | 0.405 | 0.000 |
| WBC | 0.030 | 0.766 |
| PLT | -0.276 | 0.006 |
| PT | 0.434 | 0.000 |
| INR | 0.428 | 0.000 |
| PTA | -0.431 | 0.000 |
| ALB | -0.522 | 0.000 |
| TBIL | 0.554 | 0.000 |
| DBIL | 0.602 | 0.000 |
| ALT | 0.284 | 0.004 |
| AST | 0.513 | 0.000 |
| GGT | 0.457 | 0.000 |
| ALP | 0.486 | 0.000 |
| TBA | 0.438 | 0.000 |
| HA | 0.431 | 0.000 |
| CIV | 0.493 | 0.000 |
| PIIINP | 0.511 | 0.000 |
| LN | 0.461 | 0.000 |
| CG | 0.469 | 0.000 |
| APRI | 0.532 | 0.000 |
| FIB-4 | 0.484 | 0.000 |
| King’s score | 0.543 | 0.000 |
| S-index | 0.549 | 0.000 |
| CHOL | -0.193 | 0.092 |
| TG | -0.085 | 0.464 |
| HDL | -0.215 | 0.063 |
| LDL | -0.223 | 0.053 |
| GLU | -0.099 | 0.321 |
| HBV-DNA load | 0.016 | 0.788 |
| Ascites | 0.482 | 0.000 |
| Liver cirrhosis | 0.239 | 0.016 |
| Hypersplenotrophy | 0.114 | 0.255 |
| Child-Pugh classification | 0.477 | 0.000 |
| MELD-Na score | 0.426 | 0.000 |
The Relationship Between Serum FGF19 with Laboratory and Clinical Parameters in 102 Patients with HBV-Related Liver Disease a
4.3.2. Expression of Serum FGF19 Was Associated with the Impairment of Liver Synthesis in HBV-Related Liver Disease
The liver is an essential organ for substance synthesis and metabolism, which is the only site for the synthesis of ALB and coagulation factors, including II, VII, IX, and X. When the synthetic function of the liver is impaired, there may be hypoalbuminemia, prolonged PT, decreased prothrombin activity (PTA) and increased INR. In this study, serum FGF19 levels were found to be negatively correlated with ALB and PTA but positively correlated with PT and INR, as shown in (Table 2).
4.3.3. The Levels of Serum FGF19 Could Reflect the Liver Fibrosis Stage in HBV-Related Liver Disease
Serum markers of hyaluronic acid (HA), type IV collagen (CIV), N-terminal pro-peptide of type III procollagen (PIIINP), laminin (LN), and cholyglycine (CG) are clinical indicators that can reflect the degree of liver fibrosis to a certain extent (17). Furthermore, the fibrosis index based on four factors (FIB-4), aspartate transaminase-to-platelet ratio index (APRI), King’s score, and S-index are traditional serological indexes used to indicate liver fibrosis (18-20). This study found that serum FGF19 levels positively correlated with the serum fibrosis markers mentioned above (Table 2).
4.3.4. The Relationship Between Serum FGF19 Levels with Other Parameters in Patients with HBV-Related Liver Disease
According to the correlation analysis, we also found that serum FGF19 levels had a significantly positive correlation with age, AFP, ascites, cirrhosis, Child-Pugh classification, MELD-Na score (P < 0.05), while negatively correlated with platelet count (PLT) (P < 0.05). Notably, ordered logistic regression analyses showed that age was not an independent risk factor for FGF19 elevation in patients with HBV-related liver disease (data not shown). However, no significant associations were found between serum FGF19 and blood lipid, including total cholesterol (CHOL), triglyceride (TG), high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL) (P > 0.05). In addition, there was no significant difference in serum FGF19 levels with gender, glucose (GLU), and HBV-DNA level (Table 2).
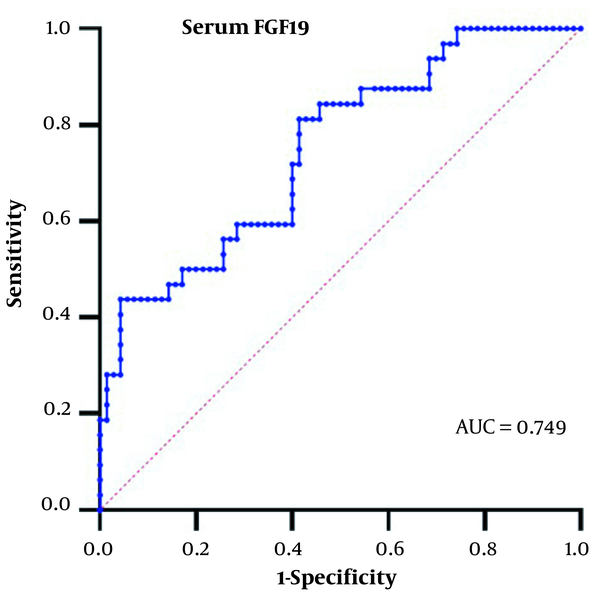
4.4. Threshold Values of Serum FGF19 in the Diagnosis of HCC for People with HBV-Related Liver Disease
Based on the significant increase of serum FGF19 in the HBV-HCC group, we analyzed the diagnostic value of serum FGF19 in HCC. We generated the area under the ROC curve in this study's HBV-related liver disease patients. The results are shown in Figure 2.
5. Discussion
The majority of liver diseases in the world are caused by chronic HBV infection. We report here that serum FGF19 levels were significantly increased in HBV-related liver disease, and the expression of serum FGF19 increased sequentially among the CHB group, HBV-cirrhosis group, and HBV-HCC group. The levels of serum FGF19 correlated with these serum indexes of liver dysfunction, liver fibrosis, etc.
FGF19/FGF15 is a hormone that modulates bile acid synthesis through negative feedback of enterohepatic circulation (3, 4, 21). It is mainly induced by the bile acid-activated nuclear receptor FXR and then enters the liver via portal vein circulation (22). Due to the toxicity of bile acids, longstanding cholestasis may lead to liver inflammation and fibrosis, even resulting in cirrhosis, liver cancer, and liver failure (23, 24). It is well-known that viral hepatitis is one of the causes of cholestasis, which aggravates the liver damage of viral hepatitis (25). This study found significant differences between serum FGF19 levels and serum markers of cholestasis, including TBIL, DBIL, GGT, ALP, and TBA. These results suggested that serum FGF19 levels have an essential role in predicting the extent of cholestasis in HBV-related liver disease. Notably, the correlation of serum FGF19 with serum markers of cholestasis is consistent with previous observations in primary biliary cirrhosis, PBC-AIH OS, and alcoholic hepatitis (5, 8, 24).
Traditional biomarkers such as ALT and AST have been widely used to detect liver injury. ALT accumulated in the cytoplasm of liver cells is released into circulation in cases of liver damage and leads to elevated serum concentrations (26). The release of mitochondrial AST from hepatocytes is evidence of hepatocyte necrosis (27). Some researchers demonstrated that serum FGF19 in primary biliary cirrhosis positively correlated with AST but not ALT (24). Another study showed that serum FGF19 levels in patients with alcoholic hepatitis were not associated with ALT and AST (5). In contrast, we observed that FGF19 positively correlated with ALT and AST in HBV-related liver disease. Still, the correlation between FGF19 and AST is stronger than that with ALT in this study, suggesting that FGF19 may be mainly regulated by mitochondrial stress and hepatocyte necrosis. Child-Pugh classification is a common index for quantitative evaluation of liver functional reserve in patients with cirrhosis (16). In addition, de Guevara et al. (28) found that the Child-Pugh classification could be used to evaluate the safety of sorafenib for HCC patients (28). MELD-Na score is another way to assess liver functional reserve and classify the degree of liver damage (29). We found a significant positive correlation between Child-Pugh classification and serum FGF19 levels in patients with cirrhosis caused by HBV infection. At the same time, there was a significant positive correlation between MELD-Na score and serum FGF19 levels in HBV-related diseases. This is consistent with the significant positive correlation between serum FGF19 and MELD score observed in PBC-AIH OS in the past (8). Because the MELD-Na score studied in this paper is a modified version of MELD. Based on these results, we speculate that serum FGF19 levels in HBV-related liver disease can be used as an indicator to predict liver functional reserve and the severity of liver disease.
The liver is an essential site for synthesizing ALB and some coagulation factors. Inadequate synthesis of coagulation factors in the liver can affect PT, INR, and PTA. In turn, the abnormal degree of PT, INR, and PTA can reflect the degree of impaired liver function. Through the relationship between serum FGF19 and ALB, PT, INR, and PTA observed in this study, we speculate that there is a specific correlation between serum FGF19 levels and the impairment of synthetic liver function caused by HBV infection. The extent of liver fibrosis is a critical decision-making factor in treating patients with CHB. Advanced fibrosis can progress to cirrhosis, liver failure, and HCC (22). Liver biopsy is a traditional reference standard for grading liver fibrosis but has invasive and sampling errors (30). Serum fibrosis markers, including HA, CIV, PIIINP, LN, CG, FIB-4, APRI, King’s score, and S-index have been proven to be noninvasive clinical indexes that can reflect the degree of liver fibrosis to some extent (17-20, 31). Dynamic changes of these markers can be used to evaluate the therapeutic effect of chronic liver disease. Previous studies reported that serum FGF19 was inversely associated with the fibrosis degree of alcoholic hepatitis and NAFLD (5, 7). The overexpression of FGF19 in mice was associated with a marked decrease in lung fibrosis and fibrosis markers (32), suggesting that FGF19 may have an anti-fibrotic effect. Another study demonstrated that serum FGF19 was not associated with the presence of cirrhosis in primary biliary cirrhosis (6). In addition, a study showed no relationship between serum FGF19 and HA, or FIB-4 in HCC (33). However, in our study, serum FGF19 levels were found to be abnormally elevated in HBV-related liver disease patients who developed cirrhosis, and there was a significant positive correlation between serum FGF19 and the serum fibrosis markers mentioned above. On the one hand, these results suggested that the serum FGF19 levels in people with HBV-related liver disease may be positively correlated with the degree of liver fibrosis; on the other hand, there may be differences in dynamic changes of serum FGF19 levels in liver diseases caused by different etiologies.
In addition, our results showed that serum FGF19 levels were significantly elevated in HBV-related liver disease patients who developed HCC and positively correlated with AFP. Although its sensitivity and specificity needed to be improved, this still suggested that serum FGF19 might be induced by carcinogenesis in humans. It is consistent with some results observed by previous studies on FGF19 and liver cancer (10, 34). Furthermore, other people revealed that serum FGF19 levels could be used to predict drug response and survival in HCC patients treated with sorafenib (35). FGF19 plays a critical role in the occurrence and development of liver carcinoma and is an indicator of an unfavorable prognosis for liver carcinoma.
However, there were some limitations in our study. Firstly, no significant differences in serum FGF19 levels between the healthy control group and the CHB group were found in this study, which may be caused by insufficient sample size and age differences. Secondly, our results did not see a specific association between serum FGF19 levels and HBV-DNA levels, indicating that serum FGF19 levels were more likely to be a response induced by immune injury but not affected directly by HBV infection. Moreover, further studies were needed to elucidate the causal relationship between serum FGF19 and HBV-related disease progression. Furthermore, there was a difference in the age between the subjects in the hepatitis group and the remaining three groups, which may have a certain impact on the results. Lastly, the pathological data of study subjects should be collected to make the results more reliable.
5.1. Conclusions
Serum FGF19 levels are associated with the severity of HBV-related liver disease and can be used as a new biological indicator to predict the progress of these diseases. Furthermore, serum FGF19 levels positively correlate with the degree of cholestasis, hepatocellular damage, and liver fibrosis but are negatively associated with liver synthesis function and liver functional reserve in HBV-related liver disease. In addition, serum FGF19 may potentially monitor carcinogenesis in patients with HBV-related liver disease. Notably, the dynamic changes of serum FGF19 levels in liver diseases caused by different etiologies may differ.