1. Context
According to reports from the International Agency for Research on Cancer (IARC) in 2021, liver cancer accounted for approximately 830,000 deaths worldwide in 2020, making it the third leading cause of cancer-related mortality (1, 2). Hepatocellular carcinoma (HCC) is the predominant form of hepatic malignancy, responsible for approximately 75% to 85% of liver cancer cases (1). Due to the lack of specific clinical symptoms in the early stages, most HCC is diagnosed at an advanced stage, requiring systemic treatment. In recent years, the field of systemic therapy for HCC has made significant progress, from Sorafenib, approved in 2007 [median overall survival (OS): 13.4 months], to the current standard first-line therapy of atezolizumab combined with bevacizumab (median OS: 19.2 months) (3), which has led to a significant improvement in the overall prognosis of HCC patients. Immune checkpoint inhibitor (ICI) therapy is making a substantial contribution to the comprehensive management of advanced HCC.
Immune checkpoint inhibitors enhance the immune system's anti-tumor activity by blocking immune downregulating factors such as programmed cell death receptor 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T lymphocyte antigen 4 (CTLA-4), thereby increasing the cytotoxicity of T-cells (4). The effect of ICIs is influenced by the immune environment of the tumor (5). It has been reported that factors such as PD-L1 expression, peripheral cytokine levels, gut microbiota, antibiotic use, growth hormone, Systemic Inflammation Response Index (SIRI), and sarcopenia can predict the prognosis of malignant tumor patients treated with ICIs (6-9), (10-12). Despite the breakthrough of ICIs combined with anti-angiogenesis therapy, the objective response rate (ORR) of ICI combination therapy for HCC is around 30% (3). Therefore, it is a major challenge to identify appropriate indications for ICI therapy by exploring predictors of ICI efficacy in serum or tissue. The presence of immune-related adverse events (irAEs) could potentially suggest enhanced effectiveness of ICI treatment (13), but biomarkers that can predict the occurrence of irAEs remain uncertain.
Alpha-fetoprotein (AFP) is a monosaccharide protein of 67 - 74 kD synthesized primarily by the liver during early fetal life. It reaches its highest level during fetal growth and decreases after birth (14). Alpha-fetoprotein, combined with imaging diagnostics, is the most widely used screening index for HCC diagnosis due to its high diagnostic specificity and sensitivity, with levels increased in approximately 70% - 80% of patients diagnosed with primary liver cancer (15). However, patients with negative AFP cannot be excluded from having primary liver cancer, as serum AFP levels are not elevated in 20% of individuals diagnosed with primary liver cancer (15). On one hand, AFP directly facilitates immune evasion by impairing the activation and function of natural killer (NK) cells. On the other hand, AFP induces abnormal differentiation of dendritic cells by inhibiting their function and reduces the generation of inflammatory cytokines and chemotactic factors, thereby limiting the activation and proliferation of T-cells, indirectly enabling immune escape (16). Alpha-fetoprotein response after treatment is an important index widely used to evaluate the efficacy of HCC treatment (17). Although baseline AFP levels can be used to predict outcomes for HCC surgery, liver transplantation, and targeted therapy (sorafenib, regorafenib) for HCC (16, 18, 19), it is unclear whether it influences ICI treatment efficacy for HCC. To examine the connection between baseline serum AFP levels and the effectiveness of ICIs in HCC, a meta-analysis was conducted. Since specific OS, progression-free survival (PFS), disease control rate (DCR), and ORR were rarely provided in the literature we searched, we also performed a retrospective cohort analysis to assess the influence of initial AFP levels on the effectiveness of ICI treatment for HCC.
2. Materials and Methods in the Meta-Analysis
This meta-analysis was conducted based on the guidelines of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) (20).
2.1. Literature Search Strategy
To ensure a thorough analysis, we conducted a comprehensive search across various electronic databases, including PubMed, Embase, Cochrane, and Web of Science, encompassing articles published prior to 14 July 2024. The ClinicalTrials.gov and Chinese Clinical Trial Registry were also screened to include updated outcomes and unpublished trials. The search terms primarily included the following words: “Immune checkpoint inhibitors,” “Pembrolizumab,” “Nivolumab,” “Atezolizumab,” “Durvalumab,” “Tislelizumab,” “Camrelizumab,” “Sintilimab,” “Carcinoma, Hepatocellular,” “Survival Rate,” “Prognosis”.
2.2. Study Selection
Inclusion criteria: (1) Study design type: Randomized controlled trials (RCTs) or cohort studies on the treatment of HCC with ICIs; (2) study subjects: Individuals diagnosed with HCC, confirmed by imaging or pathological evidence; (3) measures of intervention: Immune checkpoint inhibitormonotherapy or ICIs combined with targeted drugs; (4) study outcomes: Hazard ratios (HRs) for survival according to baseline AFP levels.
Exclusion criteria: (1) Duplicate articles; (2) articles in the following categories: Reviews, bioinformatics analyses, meeting summaries, case reports, animal experiments, expert consensuses, or editorials; (3) articles that did not specify the type of research; (4) articles that did not provide the necessary outcomes; (5) research conducted with an insufficient sample size (sample size < 100); (6) articles in languages other than English.
2.3. Data Extraction
Two independent reviewers conducted the screening and data extraction processes, and any discrepancies were resolved through discussion with a third reviewer. All reviewers were unaware of the study outcomes until the statistical analysis was performed. For each study included in the analysis, we collected the following details: Study name, year of publication, first author, study type, geographical region, sample size, demographic and baseline characteristics of participants, line of therapy, treatment strategy, clinical stage, follow-up time, demarcated value of serum AFP, number of patients at different AFP levels, and HRs for survival according to baseline AFP.
2.4. Quality Assessment
The Cochrane risk of bias evaluation tool was used to assess the quality of the RCTs, categorizing the evaluation outcomes into high, low, and unknown risk of bias. The Newcastle-Ottawa Scale (NOS) was employed to evaluate the quality of cohort studies. A total of 9 stars were used to assess article quality, and only those with fewer than 6 stars were excluded.
2.5. Statistical Analysis
The statistical analysis of relevant outcome indicators was conducted using Stata 15.1 software. The HRs for OS and PFS, along with their 95% CI (confidence intervals), were used as the summary measures, with a significance level of P < 0.05. Heterogeneity was evaluated using the Cochrane Q statistic and the I² value. A fixed-effects model was applied when I² < 50% or P > 0.10, while a random-effects model was used otherwise. Sensitivity analysis was performed using RevMan 5.3, and the risk of publication bias was assessed through Begg’s and Egger’s tests. No publication bias was considered to exist when P > 0.05. Further bias testing was not necessary if fewer than ten articles were included in the study.
3. Materials and Methods in the Retrospective Cohort Study
3.1. Patients
Patients with HCC who underwent ICI monotherapy or a combination of ICIs and targeted therapy at the Fourth Hospital of Hebei Medical University from May 7, 2019, to December 30, 2023, were included.
The following inclusion criteria had to be met: (1) Patients must be at least 18 years of age; (2) HCC was diagnosed based on the liver imaging reporting and data system (LI-RADS), AFP, and pathology, specifically LI-RADS 5, LI-RADS 4 with AFP levels > 400 ng/mL, or histopathological examination; (3) patients not suitable for radical surgical treatment; (4) patients who had not received ICIs previously; (5) patients who received at least one cycle of ICI systemic therapy and had an imaging efficacy evaluation after treatment; (6) patients with at least one measurable target lesion; (7) patients with abdominal computed tomography (CT) or magnetic resonance imaging (MRI) scan data within one week prior to ICI treatment; (8) patients with serum AFP data within one week prior to ICI treatment.
The following exclusion criteria were applied: (1) Patients with HCC combined with hepatobiliary duct carcinoma; (2) patients receiving local interventional therapy during ICI treatment; (3) patients with fatal immune-related adverse events (irAEs); (4) patients with a history of malignant tumors of other organs or liver metastases; (5) patients with incomplete electronic case data; (6) patients lost to follow-up.
3.2. Treatment
The ICIs applied include anti-PD-1 drugs (pembrolizumab, toripalimab, camrelizumab, tislelizumab, sintilimab) and anti-PD-L1 drugs (atezolizumab). The targeted drugs used include tyrosine kinase inhibitors (lenvatinib, sorafenib, regorafenib) and vascular endothelial growth factor antagonists (bevacizumab, apatinib). The dosage and administration of the drugs followed the instructions provided with the medication. Tumor response evaluation was conducted using CT or MRI scans after every 2 or 3 treatment cycles, following the guidelines outlined in version 1.1 of the response evaluation criteria in solid tumors (RECIST v1.1) (21).
3.3. Patient Outcomes
Progression-free survival was defined as the duration from the initial administration of ICI therapy until disease progression, death, or study conclusion. Overall survival was defined as the period from the commencement of ICI-based systemic treatment until death or study termination. Objective response rate was determined by calculating the proportion of patients who exhibited a complete response (CR) or partial response (PR). Disease control rate was calculated based on the percentage of patients with CR, PR, or stable disease (SD).
3.4. Variables
Alpha-fetoprotein concentrations exceeding 400 ng/mL, along with supplementary imaging, can be employed for the diagnosis of HCC (14). To evaluate the potential prognostic significance of initial serum AFP levels in predicting response to ICI therapy, patients were categorized into two groups based on a cutoff value of 400 ng/mL: A high AFP group and a low AFP group.
The clinical characteristics of each patient were also recorded, including age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS), hepatitis virus infection status, hepatitis DNA replication status, Child-Pugh score, portal vein thrombosis, number of intrahepatic lesions, maximum size of intrahepatic lesions, extrahepatic spread, Barcelona Clinic Liver Cancer (BCLC) stage, previous treatment, treatment line, treatment regimen, irAEs, and smoking history.
3.5. Statistical Analysis
Statistical analysis was conducted using IBM SPSS 15.1 software (IBM SPSS, NY, USA). The chi-square test or Fisher's exact test was used to compare categorical data. Logistic regression was utilized for multivariate analysis of categorical variables. Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test. Multivariate survival analysis was performed using a Cox proportional hazards model. A significance level below 0.05 was considered statistically significant.
4. Results
4.1. Selection Process
The two reviewers independently devised search strategies. After an initial examination, a total of 6,684 pertinent studies were identified, comprising 6,649 records from the database search and an additional 35 records from manual searching. Among these, 1,083 articles were deemed potentially relevant following title and abstract screening. Subsequent screening led to the selection of 168 articles for further evaluation. After a thorough assessment of the full texts of the remaining 168 studies, we included 34 cohort studies published between 2019 and 2024, encompassing a patient population of 8,799 individuals. Figure 1 presents a flowchart illustrating the process employed for study selection.
After screening, a total of 55 patients diagnosed with HCC and treated with ICIs at the Fourth Hospital of Hebei Medical University were included in this retrospective cohort study. The patient selection procedure is visually represented in Appendix 1 in Supplementary File.
4.2. Quality Evaluation
The Cochrane risk of bias assessment determined that the three RCTs included in this study had a minimal risk of bias (Appendix 2 in Supplementary File). The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the 30 cohort studies, and they were found to have a NOS score ≥ 6, indicating medium-to-high quality (Appendix 3 in Supplementary File).
4.3. Study and Patient Characteristics
A total of 34 enrolled articles, published between 2019 and 2024, included 30 cohort studies and 4 RCTs. Of the 30 cohort studies, 12 were from China, 5 from Japan, 2 from Korea, 2 from France, 1 from Taiwan, 1 from the USA, 1 from Singapore, and 6 were multicenter clinical studies. In 6 studies, all patients received ICI monotherapy; in 18 studies, patients were treated with immunotherapy combined with antiangiogenic therapy; in the remaining 6 studies, some patients were treated with ICI monotherapy while others received combined immunotherapy (combined with antiangiogenic therapy or locoregional therapy). All the included randomized controlled trials were phase 3 trials, including 3 global clinical trials and 1 clinical trial in China. In the 4 RCTs with sorafenib as the control group, nivolumab was used as the experimental treatment in 1 RCT, while ICI treatment combined with targeted therapy was used in 3 RCTs. The features of the selected studies are presented in Table 1 and Table 2.
| Authors | Published | Geographical Area | Research Type | Treatment Strategy | No. of Patients Total (HAFP/LAFP) | The OS HR (95%CI) for ICIs (HAFP/LAFP) | The PFS HR (95%CI) for ICIS (HAFP/LAFP) |
|---|---|---|---|---|---|---|---|
| Pinato et al. (22) | 2020 | USA/Europe/Taiwan, China | PCS | ICI monotherapy/combination ICI therapy | 341 (128/198) | 1.400 (1.1 - 2.0) | NA |
| Ng et al. (23) | 2020 | Singapore | RCS | ICI monotherapy/combination ICI therapy | 114 (53/59) | 1.420 (0.840 - 2.42) | NA |
| Fessas et al. (24) | 2020 | USA/Asia/Europe | RCS | ICI monotherapy | 233 (132/93) | 1.380 (0.96 - 2.00) | NA |
| Huang et al. (25) | 2022 | China | RCS | ICI monotherapy/combination ICI therapy | 110 (61/49) | 2.394 (0.895 - 8.400) | NA |
| Zhang et al. (26) | 2022 | China | RCS | ICI monotherapy | 101 (55/46) | NA | 3.000 (1.68 - 5.35) |
| Zhao et al. (6) | 2022 | China | RCS | Combination ICI therapy | 160 (74/86) | 1.952 (1.228 - 3.102) | 1.458 (0.965 - 2.202) |
| Song et al. (27) | 2023 | Korea | RCS | Combination ICI therapy | 208 (72/136) | NA | NA |
| Copil et al. (28) | 2023 | France | PCS | Combination ICI therapy | 293 (119/174) | 1.69 (1.23 - 2.33) | 1.29 (0.99 - 1.69) |
| Rimini et al. (29) | 2023 | Italy, Germany,Japan, and Republic of Korea | RCS | Combination ICI therapy | 761 (229/532) | 2.07 (1.55-2.75) | NA |
| Yang et al. (30) | 2023 | Korea | PCS | Combination ICI therapy | 165 (56/109) | NA | NA |
| Yang et al. (31) | 2023 | China | RCS | Combination ICI therapy | 378 (179/199) | NA | NA |
| Vithayathi et al. (32) | 2022 | Germany, Japan, Austria, United Kingdom, Italy, Taiwan and USA | RCS | Combination ICI therapy | 191 (65/126) | 1.32 (0.79 - 2.19) | NA |
| Fukushima et al. (33) | 2023 | Japan | PCS | Combination ICI therapy | 150 (-/-) | NA | NA |
| Wu et al. (34) | 2022 | Global (USA, Europe, and Asia) | RCS | Combination ICI therapy | 296 (-/-) | 1.72 (1.15 - 2.59) | 1.51 (1.11 - 2.05) |
| Yano et al. (35) | 2023 | Japan | RCS | Combination ICI therapy | 139 (45/94) | 1.416 (0.833 - 2.406) | NA |
| Uojima et al. (36) | 2023 | Japan | RCS | Combination ICI therapy | 119 (-/-) | 1.744 (0.959 - 3.170) | 1.489 (0.947 - 2.342) |
| Wang et al. (37) | 2023 | China | RCS | ICI monotherapy | 159 (68/91) | 1.326 (0.774 - 2.271) | NA |
| Khalil et al. (38) | 2023 | United States | RCS | ICI monotherapy/combination ICI therapy | 111 (30/81) | 2.35 (1.27 - 4.35) | NA |
| Zhou et al. (39) | 2023 | China | RCS | ICI monotherapy | 190 (-/-) | 1.651 (1.351 - 1.782) | 1.757 (1.271 - 1.972) |
| Chen et al. (40) | 2022 | Taiwan, China | RCS | ICI monotherapy/combination ICI therapy | 138 (52/86) | 1.43 (0.8 - 2.6) | 1.35 (0.9 - 2.04) |
| Li et al. (41) | 2024 | China | RCS | ICI monotherapy | 160 (66/94) | 1.401 (0.920 - 2.133) | 1.321 (0.943 - 1.849) |
| Du et al. (42) | 2024 | China | RCS | ICI monotherapy/combination ICI therapy | 124 (35/89) | 2.295 (1.509 - 3.491) | 1.539 (1.031 - 2.297) |
| Qin et al. (43) | 2023 | China | RCS | Combination ICI therapy | 132 (70/62) | 1.71 (1.00 - 2.91) | 1.51 (1.04 - 2.20) |
| Sultanik et al. (44) | 2024 | France | PCS | Combination ICI therapy | 200 (91/109) | 1.91 (1.32 - 2.78) | NA |
| Han et al. (45) | 2024 | China | RCS | ICI monotherapy/combination ICI therapy | 155 (30/125) | 1.409 (0.856 - 2.320) | NA |
| Suzuki et al. (46) | 2024 | Japan | RCS | Combination ICI therapy | 130 (-/-) | NA | NA |
| Kai et al. (47) | 2024 | Japan | PCS | Combination ICI therapy | 222 (-/-) | 2.307 (1.337 - 3.982) | 1.171 (0.821 - 1.671) |
| Sun et al. (48) | 2024 | China | RCS | Combination ICI therapy | 180 (68/112) | 1.59 (1.09 - 2.32) | 1.26 (0.88 - 1.8) |
| Persano et al. (49) | 2024 | Italy, Germany, Portugal, Japan and the Republic of Korea | RCS | Combination ICI therapy | 823 (-/-) | 2.128 (1.613 - 2.778) | 1.667 (1.351 - 2.041) |
| Ma, et al. (50) | 2024 | China | RCS | Combination ICI therapy | 102 (49/53) | 1.111 (0.493 - 2.564) | 1.00 (0.65 - 1.53) |
Abbreviations: HA, high AFP level; LA, low AFP level; PCS, prospective cohort study; RCS, retrospective cohort study; ICI, immune checkpoint inhibitor.
| Study | Published | Geographical Area | Research Type | Treatment Strategy | No. of Patients for HA (ICIs/NICIs) | No. of Patients for LA (ICIs/NICIs) | The OS HR (95%CI) for HA (ICIs/NICIs) | The OS HR (95%CI) for LA (ICIs/NICIs) |
|---|---|---|---|---|---|---|---|---|
| CheckMate459 (51) | 2021 | Global | RCT Phase 3 | Nivolumab vs. Sorafenib | 214 (90/124) | 390 (150/240) | 0.67 (0.51 - 0.88) | 0.98, (0.78 - 1.24) |
| ORIENT-32 (52) | 2021 | China | RCT Phase 3 | Sintilimab + Bevacizumab vs. Sorafenib | 246 (165/81) | 325 (215/110) | 0.59, (0.41 - 0.85) | 0.54, (0.35 - 0.83) |
| IMbrave150 (3) | 2022 | Global | RCT Phase 3 | Atezolizumab + Bevacizumab vs. Sorafenib | 184 (61/126) | 314 (104/210) | 0.77, (0.53 - 1.12) | 0.58, (0.42 - 0.81) |
| CARES-310 (53) | 2023 | Global | RCT Phase 3 | camrelizumab + rivoceranib vs. Sorafenib | 196 (96/100) | 347 (176/171) | 0.40, (0.28 - 0.56) | 0.66, (0.51 - 0.85) |
Abbreviations: NICIs, not immune checkpoint inhibitors; HA, high AFP level; LA, low AFP level.
The retrospective cohort study included a total of 55 patients, with 4 patients receiving ICI monotherapy and 51 patients receiving a combination of ICIs and targeted therapy. Among them, there were 28 patients in the high AFP group (AFP ≥ 400 ng/mL) and 27 patients in the low AFP group (AFP < 400 ng/mL). The characteristics of the patient population can be found in Appendix 4 in Supplementary File. The overall median OS was 13.967 months (95% CI: 14.618 - 23.316), while the median PFS was 7.267 months (95% CI: 2.522 - 12.012). In terms of clinical efficacy assessment, none of the patients achieved CR, but PR was observed in 3 cases, and SD was seen in 30 cases, resulting in an ORR of 5.6% (95% CI: -0.7% - 11.7%) and a DCR of 60% (95% CI: 46.6%-73.4%) (Table 3).
| Response | Total | High AFP | Low AFP | P-Value |
|---|---|---|---|---|
| PD | 22 | 16 | 6 | - |
| SD | 30 | 11 | 19 | - |
| PR | 3 | 1 | 2 | - |
| CR | 0 | 0 | 0 | - |
| ORR | 5.6% (95% CI: -0.7% - 11.7%) | 3.6% (95% CI: -3.8% - 10.9%) | 7.4% (95% CI: -3.2% - 18.0%) | 0.611 |
| DCR | 60.0% (95% CI: 46.6% - 73.4%) | 42.9% (95%CI: 23.3% - 62.4%) | 77.8% (95% CI: 61.0% - 94.5%) | 0.008 |
4.4. Evaluation of Survival Outcomes
4.4.1. Hazard Ratios of Alpha-fetoprotein ≥ 400 ng/mL vs. Alpha-fetoprotein < 400 ng/mL for Immune Checkpoint Inhibitors Therapy
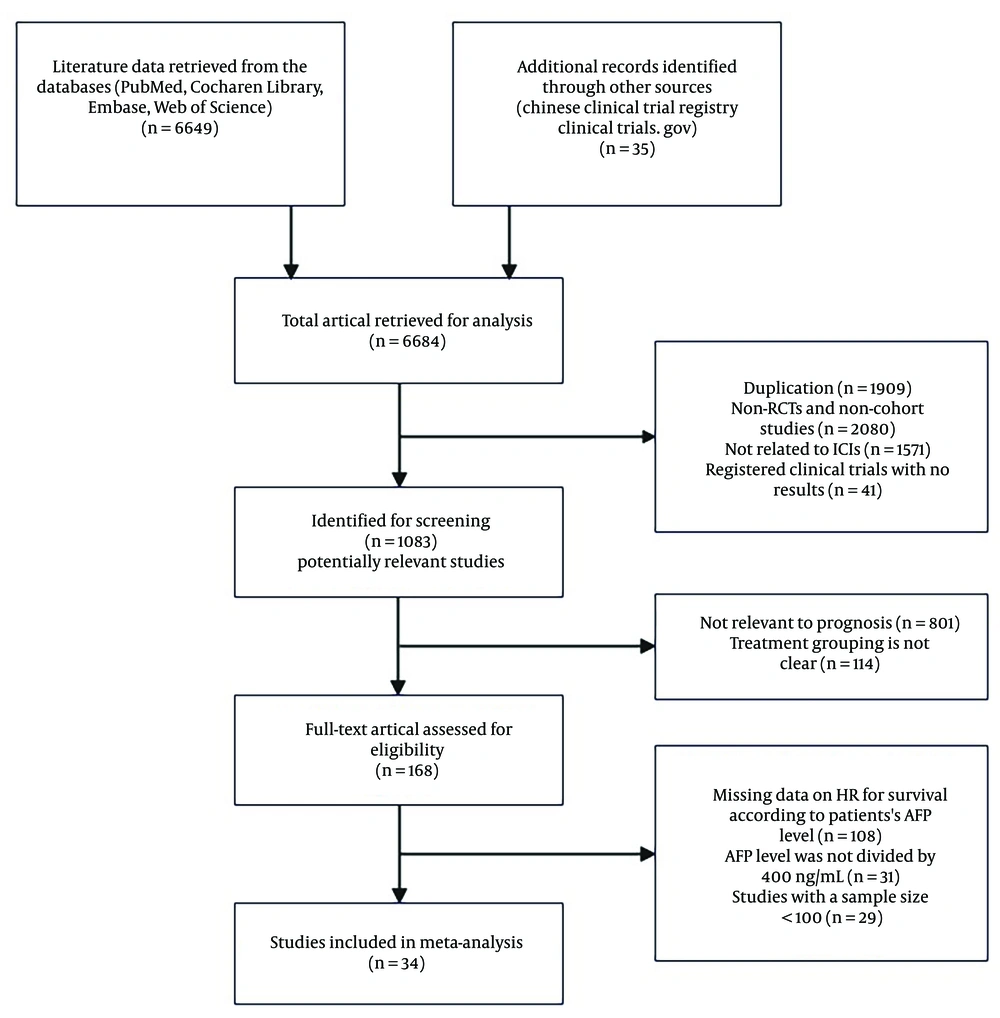
In the 24 cohort studies that provided HRs for AFP ≥ 400 ng/mL vs. AFP < 400 ng/mL for OS in univariate analysis (6, 22, 28, 29, 32, 34), the combined HR for OS was 1.69 (95% CI: 1.57-1.82, P < 0.001), indicating low heterogeneity (I² = 0.0%, P = 0.743) (Figure 2A). This suggests that higher levels of AFP are significantly associated with poorer survival outcomes compared to lower AFP levels after ICIs therapy. The high AFP group had a 1.69-fold increased risk of mortality compared to the low AFP group. In the 17 cohort studies (6, 13, 16, 29, 32, 34), (25, 27, 30, 31, 33, 39, 42-44, 47-49) that included multivariate analysis for OS outcomes, the combined adjusted HR for OS was found to be 1.62 (95% CI: 1.44 - 1.81, P < 0.001) with low heterogeneity (I² = 17.3%, P = 0.251) (Figure 2B), indicating a significant association between elevated AFP levels and increased mortality following ICIs therapy.
In the subgroup analysis, the combined HR for OS was found to be 1.58 (95% CI: 1.40 - 1.78, P < 0.001) (Figure 2C) for the ICIs monotherapy group (24, 37, 39, 41) and 1.84 (95% CI: 1.64 - 2.07, P < 0.001) (Figure 2D) for the ICIs therapy combined with antiangiogenic therapy group (6, 22, 28, 29, 32, 34). The results indicated that patients with elevated AFP levels had a greater likelihood of mortality, regardless of whether they received ICIs alone or in combination with antiangiogenic therapy. The negative effect of high serum AFP levels on survival outcomes was not significantly different between ICIs monotherapy and ICIs therapy combined with antiangiogenic therapy (P = 0.096).
Hazard ratios (HRs) of OS for alpha-fetoprotein (AFP) ≥ 400 ng/mL vs. AFP < 400 ng/mL after immune checkpoint inhibitors (ICIs) treatment, in 28 cohort studies. Squares indicated study-specific HRs. 95% confidence intervals are depicted by horizontal lines. Diamonds symbolize the combined HRs. The dotted vertical lines represent the HRs pooled. The P-value for heterogeneity is derived from the meta-analysis of the interaction. A, pooled HR of overall survival (OS) on univariate analysis; B, pooled HR of OS on multivariate analysis; C, pooled HR of OS for the ICIs monotherapy group; D, pooled HR of OS for the ICIs therapy combined with antiangiogenic therapy group.
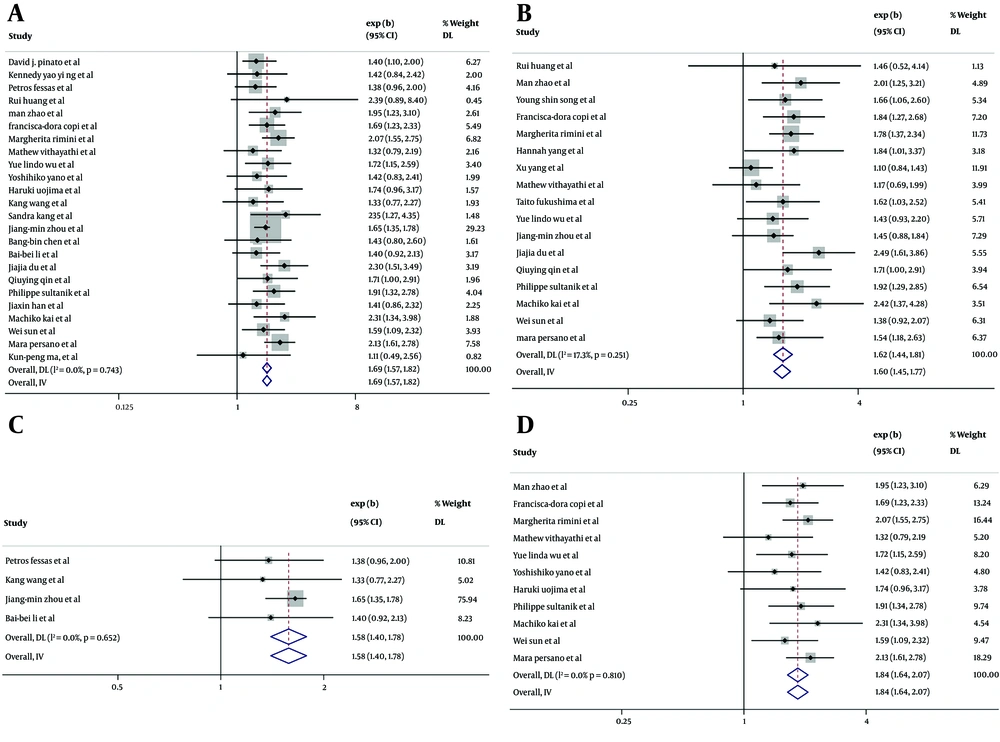
In the 14 cohort studies that provided HRs for AFP ≥ 400 ng/mL vs. AFP < 400 ng/mL for PFS in univariate analysis (6, 26, 28, 34, 36, 39-43, 47-50), the combined HR for PFS was found to be 1.47 (95% CI: 1.33-1.63, P < 0.001) with low heterogeneity (I² = 22.3%, P = 0.212) (Figure 3A). This indicates that the high AFP group had a 1.47 times greater likelihood of progression compared to the low AFP group. In the 12 cohort studies that provided HRs for PFS in multivariate analysis (6, 26, 27, 30, 31, 33, 34, 39, 42, 43, 46), the combined adjusted HR for PFS was found to be 1.52 (95% CI: 1.33 - 1.73, P < 0.001) with low heterogeneity (I² = 36.9%, P = 0.096) (Figure 3B), suggesting that higher AFP was independently associated with a higher risk of progression after ICI therapy. The combined HR for PFS was 1.57 (95% CI: 1.19 - 2.06, P = 0.001) (Figure 3C) for the ICIs monotherapy group (39, 41), and 1.44 (95% CI: 1.27 - 1.62, P < 0.001) (Figure 3D) for the ICIs therapy combined with antiangiogenic therapy group (28, 34, 38, 47-49). These findings indicate that patients with elevated AFP levels have a greater likelihood of disease progression, regardless of whether they receive ICIs alone or in combination with antiangiogenic therapy. The negative effect of high serum AFP levels on the risk of progression was not significantly different between ICIs monotherapy and ICIs therapy combined with antiangiogenic therapy (P = 0.532).
Hazard ratios (HRs) of progression-free survival (PFS) for alpha-fetoprotein (AFP) ≥ 400 ng/mL vs. AFP < 400 ng/mL after immune checkpoint inhibitors (ICIs) treatment, in 19 cohort studies. Squares indicated study-specific HRs. 95% confidence intervals are depicted by horizontal lines. Diamonds symbolize the combined HRs. The dotted vertical lines represent the HRs pooled. The P-value for heterogeneity is derived from the meta-analysis of the interaction. A, combined HR of PFS on univariate analysis; B, combined HR of PFS on multivariate analysis; C, combined HR of PFS for the ICIs monotherapy group; D, combined HR of PFS for the ICIs therapy combined with antiangiogenic therapy group.
4.4.2. Hazard Ratios of Immune Checkpoint Inhibitors Therapy vs. Targeted Therapy for Alpha-fetoprotein
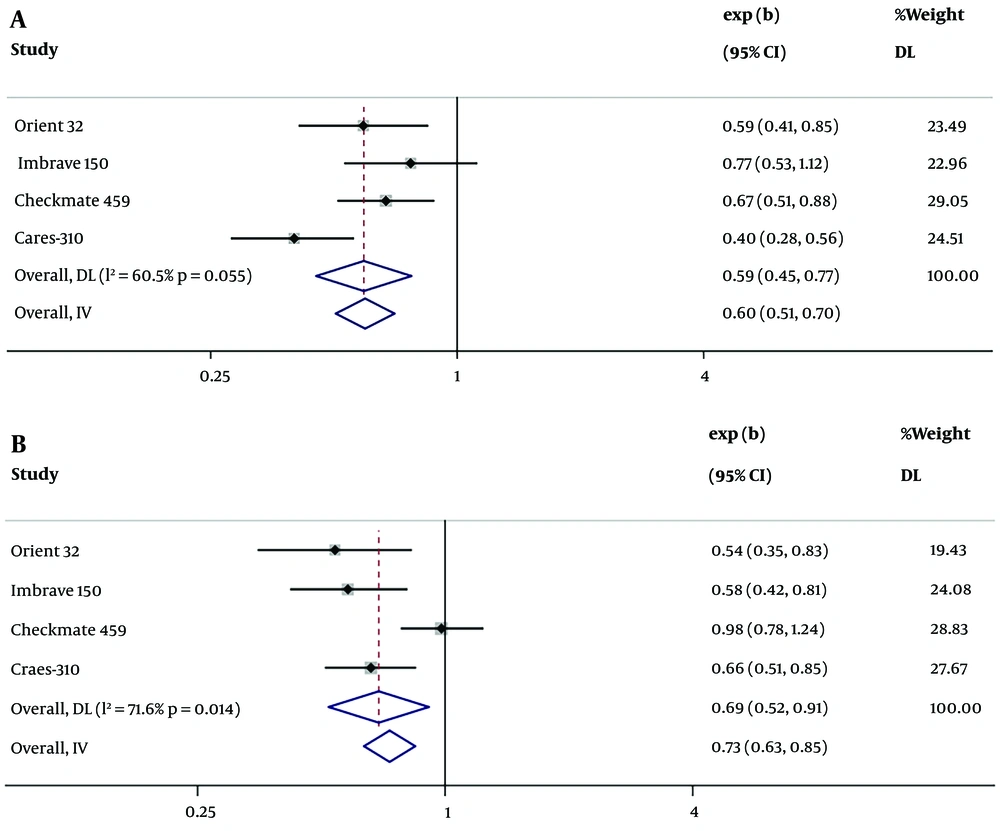
The 4 included RCTs (3, 51-53) provided HRs for OS comparing ICIs therapy to targeted therapy. For patients with high AFP levels (AFP ≥ 400 ng/mL), the mortality rate was found to be lower in those who received ICIs therapy compared to those who underwent targeted therapy, with the combined HR for the high AFP group (AFP ≥ 400 ng/mL) being 0.60 (95% CI: 0.51 - 0.70, P < 0.001) and high heterogeneity (I² = 60.5%, P = 0.055) (Figure 4A). The combined HR for the low AFP group (AFP < 400 ng/mL) was 0.73 (95% CI: 0.63 - 0.85, P < 0.001) with high heterogeneity (I² = 71.6%, P = 0.14) (Figure 4B). The heterogeneity may be caused by the different drug effects in the experimental groups across the RCTs. There was no significant difference in the HR of ICIs therapy versus targeted therapy between the high AFP group and the low AFP group (P = 0.456), suggesting that ICIs therapy has better efficacy than targeted monotherapy in both the high AFP and low AFP groups.
Hazard ratios (HRs) of immune checkpoint inhibitors (ICIs) therapy vs. targeted therapy for alpha-fetoprotein (AFP), in 4 randomized controlled trials (RCTs). Squares indicated study-specific HRs. 95% confidence intervals are depicted by horizontal lines. Diamonds symbolize the combined HRs. The dotted vertical lines represent the HRs pooled. The P-value for heterogeneity is derived from the meta-analysis of the interaction. A, pooled HR of overall survival (OS) for AFP ≥ 400ng/mL in 4 RCTs; B, pooled HR of OS AFP < 400 ng/mL in 4 RCTs.
4.4.3. Progression-Free Survival and Overall Survival in the Retrospective Cohort Study
In the retrospective cohort study, the clinical characteristics that may affect PFS in HCC patients were analyzed using univariate analysis. As shown in Appendix 5 in Supplementary File, the high AFP group exhibited a significantly shorter median PFS (2.467 months, 95% CI: 1.345 - 3.589) compared to the low AFP group (15.600 months, 95% CI: 4.203 - 26.997). In the multivariate analysis, no statistically significant difference in PFS was observed between the high and low AFP groups (HR 1.822, 95% CI: 0.866 - 3.832, P = 0.114). The Kaplan-Meier curve for PFS is shown in Appendix 7A in Supplementary File.
In the univariate analysis of OS, the low AFP group exhibited a median OS of 21.800 months (95% CI: 11.935 - 31.665), demonstrating significantly longer survival compared to the high AFP group, which had an OS of 7.300 months (95% CI: 5.443 - 9.157), at a marginally significant statistical level (HR: 2.119, 95% CI: 0.988 - 4.544, P = 0.054, Appendix 6 in Supplementary File). In the multivariate analysis, high AFP was independently associated with shorter OS in HCC patients after ICI treatment (HR: 3.584, 95% CI: 1.466 - 8.762, P = 0.005, Appendix 6 in Supplementary File). In summary, serum AFP level serves as an independent indicator for predicting survival in HCC patients following ICI treatment, with the high AFP group exhibiting a 2.119 times greater risk of mortality compared to the low AFP group. The Kaplan-Meier curve for OS is shown in Appendix 7B in Supplementary File.
4.5. Evaluation of Response to Treatment
There was no significant difference observed in the ORR between the two cohorts (P = 0.611), with 3.6% (95% CI: -3.8% to 10.9%) in the high AFP group and 7.4% (95% CI: -3.2% to 18.0%) in the low AFP group. However, the DCR distribution was significantly different, with 42.9% (95% CI: 23.3% - 62.4%) for the high AFP group and 77.8% (95% CI: 61.0% - 94.5%) for the low AFP group (P = 0.008, Table 3). These findings suggest that the effectiveness of ICIs in HCC may be influenced by the presence of serum AFP.
4.6. Toxicity Analysis in the Retrospective Cohort Study
No literature was found providing information on the incidence of irAEs associated with AFP levels. Therefore, we analyzed the relationship between AFP levels and the occurrence of irAEs in retrospective data. During the follow-up period, irAEs were observed in 19 patients (9 with thyroid dysfunction, 3 with myocarditis, 3 with enteritis, 3 with dermatitis, 3 with pneumonia, 2 with hepatitis, 2 with myositis, and 1 with thrombocytopenia). Serum AFP levels were correlated with the occurrence of irAEs (P = 0.008, Appendix 4 in Supplementary File). High AFP levels were significantly associated with a lower incidence of irAEs in the univariate analysis (OR: 0.202, 95% CI: 0.059 - 0.688, P = 0.011). In the multivariate analysis, a significant independent correlation was observed between these two clinical characteristics (OR: 0.210, 95% CI: 0.045 - 0.971, P = 0.046).
4.7. Sensitivity Analysis and Publication Bias
To assess the robustness and reliability of the computed outcomes, a sensitivity analysis was conducted. The findings indicate that the exclusion of any study in this analysis does not affect the overall results (Appendix 8 in Supplementary File).
According to the results of Begg's and Egger’s tests (Appendix 9 in Supplementary File), there is no evidence of publication bias in this study.
5. Discussion
There is no doubt that AFP has value in evaluating the prognosis of HCC, but its full application scope remains unclear. Alpha-fetoprotein ≥ 100 ng/mL has been reported to be associated with shorter OS in HCC patients treated with atezolizumab in combination with bevacizumab (54), but few studies have explored the correlation between AFP and ICI efficacy, including DCR, ORR, and PFS in HCC patients. The main finding of our meta-analysis is that baseline serum AFP ≥ 400 ng/mL is associated with a higher risk of death and progression in HCC patients treated with ICIs, making it a potential predictor of ICI treatment efficiency in these patients.
We also compared treatment groups, finding that patients in the high AFP group had a higher risk of death and progression regardless of whether they were treated with ICIs monotherapy or ICIs combined with antiangiogenic therapy, with no significant difference in the predictive effect of high AFP levels on the negative outcomes of the two treatments. The analysis of 4 RCTs suggests that even in the high AFP group, ICI therapy, especially ICIs combined with antiangiogenic therapy, is superior to targeted monotherapy.
We comprehensively compared the effect of baseline AFP levels on clinical outcomes across different treatment groups, including ICIs monotherapy, ICIs combined with antiangiogenic therapy, and targeted therapy, and this result has not been previously reported. The main finding of our retrospective cohort study is that baseline serum AFP ≥ 400 ng/mL is associated with both lower DCR and poorer survival outcomes. We reported for the first time the correlation between baseline serum AFP levels and DCR, further explaining the association between baseline serum AFP levels and the prognosis of ICIs treatment.
Alpha-fetoprotein binds to the AFP receptor (AFPR), activating the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway (55-57). This pathway has the potential to increase the expression of vascular endothelial growth factor (VEGF), a key mediator in hepatocarcinogenesis. It achieves this by promoting the formation of new blood vessels, ultimately leading to the invasion and metastasis of HCC (55, 58). Vascular endothelial growth factor reduces the therapeutic effect of ICIs by inhibiting dendritic cell maturation, intra-tumoral T-cell infiltration, and the expansion of immunosuppressive myeloid-derived suppressor cells (54, 58-60). Vascular endothelial growth factor may also hinder the effectiveness of ICI therapy on tumors by promoting the growth and viability of endothelial cells (ECs), resulting in the formation of numerous abnormal and dysfunctional neovessels within the tumor (61). Additionally, AFP can disrupt the establishment of anti-cancer immunity by directly inhibiting dendritic cells, affecting T-cell activation (16). These factors may partly explain the mechanism behind AFP-related reductions in ICI treatment efficacy in HCC patients. Our next objective is to further investigate the impact of AFP on immune cells and cytokines within the tumor microenvironment through basic experiments, aiming to better understand its effect on the efficacy of ICIs.
Immune checkpoint inhibitors and antiangiogenic drugs are currently widely used in the systemic treatment of HCC. It has been reported that the prognosis of targeted therapy for AFP-related HCC is poor (16, 18, 19). In this study, we found that the efficacy of ICIs monotherapy and ICIs combined with targeted therapy for AFP-related HCC was lower compared to non-AFP-related HCC. Given the limited therapeutic efficacy of the current standard systemic treatments, down-regulating AFP expression may be an ideal target for treating AFP-related HCC. As a result, some AFP vaccines and engineered T-cell therapies targeting AFP are currently being evaluated in clinical trials (60, 62).
High AFP was independently associated with a lower incidence of irAEs in HCC patients, according to our retrospective cohort analysis. This complements our previous findings that irAEs are associated with better prognosis in ICIs therapy (13). The possible mechanism for the negative association between AFP and irAEs may be that AFP antagonizes the production of inflammatory factors involved in irAEs initiation. Most irAEs are categorized as autoimmune disorders caused by CD8+ cytotoxic T-cells activated by ICIs. Alpha-fetoprotein could potentially hinder T-cell-dependent immune functions and modify the CD4+ T/CD8+ T-cell ratio, thereby reducing the incidence of irAEs (63-65). In some irAEs evaluated with interleukin 6 (IL-6), AFP may reduce the occurrence of irAEs by inhibiting IL-6 production (65, 66).
5.1. Limitations
There are some limitations to this study. Firstly, some of the included studies were retrospective cohort studies, which come with inherent limitations and inevitable selection bias. Secondly, the retrospective analysis in this study included a relatively small number of patients. Thirdly, this study excluded certain factors that may cause fluctuations in AFP levels in HCC, such as smoking and other malignant tumors; however, it did not account for factors like HBV-DNA replication status, which could reflect viral flares. Additionally, although the review was not officially registered, we conducted the meta-analysis in strict adherence to the guidelines outlined in the PRISMA statement. Given these limitations, it is crucial to conduct multi-center, high-quality clinical studies with a substantial sample size to further advance our research.
5.2. Conclusions
Higher baseline serum AFP levels were significantly associated with poorer clinical outcomes in HCC patients treated with ICIs, whether as monotherapy or in combination with targeted therapy. Targeting AFP therapy may represent a new breakthrough in the systemic treatment of AFP-related HCC.