1. Background
Hepatocellular adenoma (HCA) is a benign hepatic tumor, with a high incidence in women of child bearing age. This tumor has different risk factors such as history of oral contraceptive drug (OCP) and androgen use, obesity, diabetes and glycogen storage disease (1). In the past, HCA used to be considered as a completely benign tumor with no malignant potential; however, it has now been proved that HCAs are heterogeneous regarding complications such as malignant potential and hemorrhage. In some subtypes, there is a 4% - 8% risk of malignant transformation and in others there is high risk of hemorrhage. According to the WHO (World health organization) classification of liver tumors (2010), HCAs are classified into 4 subtypes with different molecular pathogeneses (2).
These four most popular and accepted subtypes are as below (3):
1- Hepatocyte nuclear factor-1α (HNF1α) inactivated type, which is called H-HCA. These HCAs are negative for liver fatty acid binding protein (LFABP).
2- β-catenin activated type, which is called b-HCA. These cases show positive nuclear β-catenin or/and positive glutamine synthetase. Some of these cases show mutation in exon 3 with strong Wnt pathway activation and rare forms show mutation in exon 7 and 8 with weak Wnt pathway activation.
3- Inflammatory type which is called I-HCA. These are positive for CRP (C-reactive protein) or/and serum amyloid A (SAA).
4- Unclassified (U-HCA). These are cases which can not be categorized in the abovementioned 3 subtypes. Recently in some of the cases of this subtype activation of the sonic hedgehog pathway has been demonstrated (4).
2. Objectives
To the best of our knowledge, there has been no study about HCA and its subtypes from Iran and also this classification has rarely been studied in other Asian countries, so in this study we tried to evaluate different subtypes of 40 cases of HCA during 10 years (2008-2018) in the largest hepatobiliary referral center in the south of Iran.
3. Methods
In this cross-sectional study, during 10 years (2008 - 2018), we extracted all (40) cases with the pathologic diagnosis of hepatocellular adenoma from the archives of pathology department in the affiliated hospitals of Shiraz University of medical Sciences.
All the pathology slides were reviewed by two pathologists (BG&ZM), the diagnosis was confirmed and histopathologic findings were recorded. In the meantime, the best Hematoxylin & Eosin (H&E) slide was selected to extract the related paraffin block and to perform immunohistochemistry (IHC) on a freshly cut slide. Among the abovementioned 40 cases, 15 specimens were tumor resected tissue by surgery and 25 cases were needle biopsies.
We also evaluated the clinical charts of the patients to find out about the main clinical findings, such as drug history (mainly OCP and steroid androgens), obesity and diabetes mellitus (DM).
In this study we evaluated 4 IHC markers i.e. LFABP, β-catenin, Serum amyloid A (SAA), and glutamine synthetase (GS) which are the most recommended IHC markers in the very recent literature (1). Table 1 shows the characteristics of the antibodies and the methodology for IHC staining.
| Antibody | Source | Clone | Antigen retrieval | Dilution | Company |
|---|---|---|---|---|---|
| Glutamine synthetase | Mouse | GS-6 | Trypsin | 1/100 | Medysis |
| Serum amyloid A | Rabbit | EP335 | Citrate | 1/200 | Medysis |
| Liver fatty acid binding protein | Mouse | F9 | Citrate | 1/200 | Medysis |
| Beta-catenin | Mouse | 14 | Heat | 1/200 | Biocare |
4. Results
A total of 40 HCA cases (15 resections and 25 biopsies) were included in this study (male = 5, female = 35, mean age 33 ± 17 years).
Fifteen patients (37.5%) had multiple adenomas. The average size of HCA was 8.6 ± 6.8 cm (range: 2 - 17 cm).
Table 2 shows the details of clinicopathologic findings in these 40 cases. Among the female patients, use of oral contraceptives (OCP) for at least 6 months, was identified in 22 of 35 cases (63%) cases. One of 5 male patients (20%) reported anabolic steroid use (for the duration 2 years). Eight patients (20%) were diabetic. It’s worth mentioning that 7 patients with diabetes also had the history of OCP usage. There were 3 patients with a body mass index (BMI) more than 25.
| Case | Gender/Age, y | Size, largest/cm | Numbers | Additional Findings | Main Histologic Finding | Adenoma Subtype by IHC |
|---|---|---|---|---|---|---|
| 1 | F/30 | 2 | 1 | OCP | Hemorrhage | U-HCA |
| 2 | F/33 | 13 | 2 | OCP | Hemorrhage | I-HCA |
| 3 | F/32 | 10 | 1 | - | Atypia and Acini | b-HCA |
| 4 | F/38 | 4 | 1 | BMI > 25 kg/m2 | Steatosis | H-HCA |
| 5 | F/43 | 9 | 3 | OCP and DM | Hemorrhage | U-HCA |
| 6 | F/26 | 4 | 1 | - | Steatosis and inflammation | H-HCA |
| 7 | M/59 | 17 | 1 | Androgen | Atypia and Acini | b-HCA |
| 8 | M/36 | 4.5 | 1 | - | Steatosis | H-HCA |
| 9 | F/35 | 14 | 1 | OCP | Steatosis | H-HCA |
| 10 | F/34 | 7 | 1 | OCP and DM | Steatosis | H-HCA |
| 11 | F/33 | 4 | 1 | OCP | Steatosis | H-HCA |
| 12 | F/28 | 2.5 | 1 | OCP | Steatosis and inflammation | I-HCA |
| 13 | F/19 | 4 | 2 | - | Inflammation | U-HCA |
| 14 | F/38 | 14 | 4 | OCP | Steatosis | H-HCA |
| 15 | F/43 | 4 | 2 | OCP | Steatosis | H-HCA |
| 16 | F/40 | 13 | 2 | OCP and DM | Steatosis | H-HCA |
| 17 | F/28 | 4 | 1 | OCP and BMI > 25 kg/m2 | Steatosis | H-HCA |
| 18 | F/31 | 10 | 1 | OCP | Hemorrhage | U-HCA |
| 19 | F/35 | 11 | 1 | OCP | Steatosis | H-HCA |
| 20 | F/42 | 10 | 1 | OCP | Steatosis | H-HCA |
| 21 | F/37 | 16 | 2 | OCP | Steatosis | H-HCA |
| 22 | F/48 | 4 | 2 | OCP | Steatosis | H-HCA |
| 23 | F/19 | 6 | 2 | - | Hemorrhage | U-HCA |
| 24 | M/17 | 2 | 1 | - | Steatosis | H-HCA |
| 25 | F/53 | 15 | 2 | OCP and DM | Inflammation and Hemorrhage | I-HCA |
| 26 | F/39 | 9 | 1 | OCP and DM | Steatosis | H-HCA |
| 27 | F/40 | 10 | 1 | OCP and DM | Atypia | b-HCA |
| 28 | F/26 | 6 | 3 | hemorrhage | Hemorrhage | U-HCA |
| 29 | F/37 | 9 | 1 | OCP and DM | Steatosis | H-HCA |
| 30 | F/42 | 2 | 1 | - | Steatosis | H-HCA |
| 31 | F/19 | 6 | 2 | DM and BMI > 25 kg/m2 | Hemorrhage | U-HCA |
| 32 | F/36 | 6 | 1 | OCP | Inflammation | I-HCA |
| 33 | F/16 | 6 | 2 | - | Inflammation | I-HCA |
| 34 | F/37 | 9 | 1 | OCP | Inflammation | I-HCA |
| 35 | M/30 | 3 | 1 | - | Steatosis | H-HCA |
| 36 | M/17 | 11 | 1 | - | Atypia | b-HCA |
| 37 | F/3 | 6 | 2 | GSD | Inflammation and Hemorrhage | I-HCA |
| 38 | F/16 | 6 | 1 | - | Steatosis | H-HCA |
| 39 | F/9 | 5 | 1 | - | Inflammation | I-HCA |
| 40 | F/63 | 4 | 3 | - | Hemorrhage | U-HCA |
Abbreviations: BMI, body mass index; b-HCA: β-catenin mutated hepatocellular adenoma; DM, diabetes Mellitus; GSD, glycogen storage disease; H-HCA, hepatocyte nuclear factor mutated hepatocellular adenoma; I-HCA, inflammatory hepatocellular adenoma; OCP, Oral contraceptive pill; U-HCA, unclassified hepatocellular adenoma.
We identified one case of glycogen storage disease in a 3-year old child with HCA.
By applying the IHC criteria (2, 3), we identified 4 (10%) b-HCA, 8 (20%) I-HCA and 20 (50%) H-HCA and 8 cases (20%) were negative for β-catenin, GS, SAA, and LFABP, and therefore were unclassifiable (U-HCA) (Tables 2 and 3).
| Glutamine Synthetase | β-Catenin | Serum Amyloid A | Liver Fatty Acid Binding Protein | No. (%) | |
|---|---|---|---|---|---|
| H-HCA | - | - | - | - | 20 (50) |
| I-HCA | - | - | + | + | 8(20) |
| b-HCA | + | + | - | + | 4(10) |
| U-HCA | - | - | - | + | 8(20) |
Abbreviations: b-HCA: β-catenin mutated hepatocellular adenoma; H-HCA, hepatocyte nuclear factor mutated hepatocellular adenoma; I-HCA, inflammatory hepatocellular adenoma; U-HCA, unclassified hepatocellular adenoma.
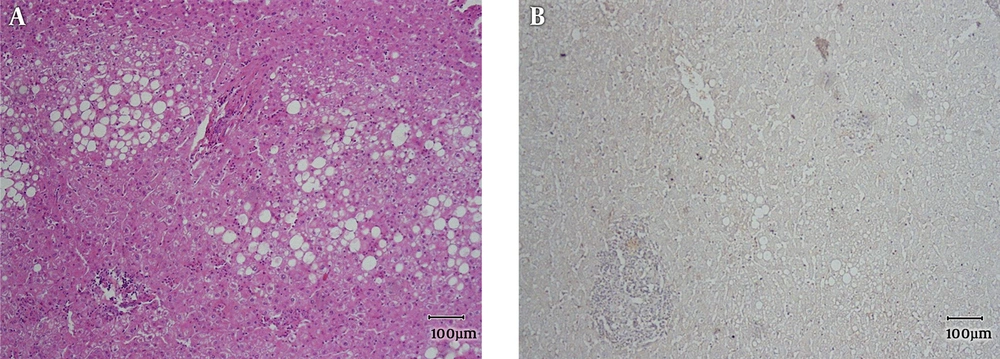
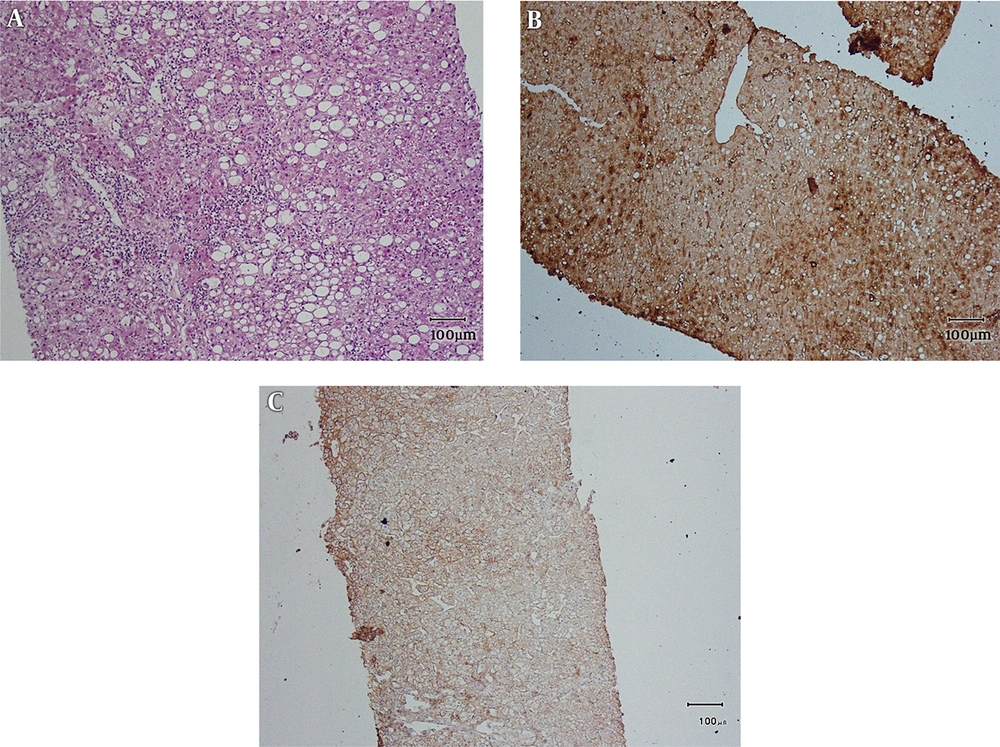
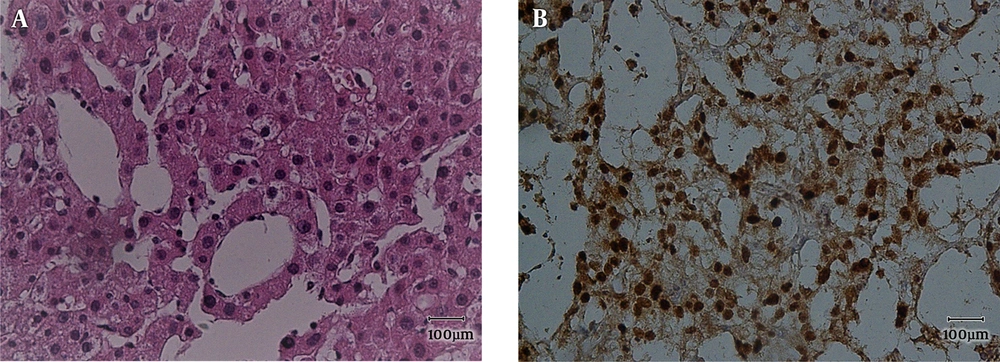
Main histopathologic findings were inflammation in 9 cases (22.5%), hemorrhage in 10 cases (25%), steatosis in 20 cases (50%), acinar transformation in 2 cases (5%) and atypia in 4 cases (10%). Inflammation and hemorrhage were seen together in 2 cases. Steatosis and inflammation were seen together in 2 cases. All of the cases with atypia were β-catenin positive. Two of them were male, one with the history of androgen use and size of larger than 5 cm (Figures 1 - 4).
All of the 20 cases of H-HCA showed steatosis, one of which was also inflamed. In eight cases of I-HCA, 7 cases showed inflammation, in one of which inflammation was associated with steatosis and two with hemorrhage. Three cases showed hemorrhage, two of which were associated with inflammation. In U-HCA cases, 7 showed hemorrhage and one showed inflammation.
5. Discussion
HCA shows variable pathophysiologic pathways of development which contributes to different prognoses and varying malignant potential (1). This is because of different molecular and immunohistochemical characteristics, which result in different management protocols. According to WHO, varying protein expression and also histomorphologic findings determine malignant potential and prognosis of these tumors (2). In some studies immunohistochemistry has been the base of classification and in some others, molecular methods have been used to classify HCA (4-16). Another possible reason for the different results in the frequencies has been because of the variety of geographic regions and ethnic population of the studies. The most important point to classify HCAs according to their molecular and immunohistochemical findings is to separate those with malignant potential to perform special procedures. However, as Table 4 shows there have been many controversial reports about the percentage of β-catenin mutated HCAs. Studies from the USA report the results of b-HCA from zero (2) to 15.4% (14) which can be secondary to variable ethnicity in this country. Studies from Japan are more consistent and two reported studies have had similar results around 15% (12, 13). There has not been any report from Iran about the molecular and immunohistochemical classification of HCA. We decided to report our experience about HCA and immunohistochemical classification from our center as the largest referral hepatobiliary center in the south of country.
Abbreviations: b-HCA: β-catenin mutated hepatocellular adenoma; H-HCA, hepatocyte nuclear factor mutated hepatocellular adenoma; I-HCA, inflammatory hepatocellular adenoma; U-HCA, unclassified hepatocellular adenoma.
aValues are expressed as percentage.
As Tables 2 and 3 show, the most common subtype of HCA in our center has been type I or H-HCA. About half of the 40 cases showed negative LFABP which correlates with the presence of fat and classifies the tumor in the first category. The least common subtype in our study has been β-catenin mutated type which has the higher risk of malignancy. As Table 4 shows the reported frequencies from Japan, Australia, France and USA have been very different and, in some studies, mixed I-HCA and b-HCA has been very common (2, 15).
In most of the previous reports, the most common subtype has been inflammatory (I-HCA), but in our study the most common subtype has been H-HCA. It’s the subtype which most commonly occurs in the patients with the history of OCP ingestion (1). In our study more than 50% of patients had the history of OCP use.
As mentioned above, Table 4 shows even studies from the same country reporting different frequencies. This means that the issue is evolving and the difference in the frequencies is multifactorial, which emphasizes the importance of routine classification of the cases with the diagnosis of HCA (4, 12-17).
Management of HCAs is mostly based on the size (more than 5 cm) and the presence or absence of β-catenin mutation (1). Our population has been amongst the lowest number of the cases with β-catenin mutation (10%), and studies from USA have shown up to 50% HCAs with the mutation of β-catenin, either pure or mixed with inflammatory pathogenesis (15). In our cases, all of those with β-catenin activated HCAs (b-HCA) showed different degrees of cellular atypia with and without acinar transformation. There has also been different degrees of steatosis and inflammation in the cases of I-HCA and H-HCA. This finding correlates with previous reports, which have shown steatosis in all of the subtypes of HCA (6-9).
5.1. Conclusions
HCA should be considered as a heterogenous tumor in the diagnosis of which IHC subclassification should be part of routine practice. Frequencies of different subtypes are completely different in different populations. Histologic findings are informative but not adequate for subclassification of HCA. Further cohort studies are necessary for definite evaluation of the role of IHC study in subclassification and long-term prognosis of HCA.