1. Background
The hepatitis B virus (HBV) is one of the major causes of chronic liver disease. There is an effective vaccine against the virus, and oral antivirals can control the disease. However, the molecular pathogenesis of the disease precludes a definite cure (1, 2). The covalently closed circular DNA integrated into the hepatocyte genome cannot be eradicated (2). Globally, an estimated 257 million people are infected with HBV based on current data, and 887,000 people die annually from chronic hepatitis B and related complications (3). In a systematic review of the studies conducted between 1999 and 2009 in the Republic of Turkey in terms of age and region, it was reported that approximately 3.3 million people (4.6%) with HBsAg positivity were infected with chronic HBV (4).
From the perspective of hospital workers (HWs), employees are at risk of hepatitis B infection because of the possibility of occupational exposure. Apart from this occupational risk, health professionals may still be affected by HBV, depending on the epidemiological characteristics of the countries and geographical regions they live in (5, 6). Taking the hepatitis B vaccine is mandatory for health professionals, according to the recommendations of the Centres for Disease Control and Prevention (7). In Turkey, the hepatitis B vaccine is included in the list of vaccines supplied by the Ministry of Health for HWs (7-9).
The World Health Organization (WHO) estimated that in 2016, approximately 399,000 people died from hepatitis C, mainly from cirrhosis and hepatocellular carcinoma (10). The hepatitis C virus (HCV) is mainly transmitted by contact with infected blood due to injuries to the skin or mucous membranes (2). There is no vaccine or postexposure prophylaxis for HCV infection (11). Although HCV infection as an occupational disease is statistically rare, as HWs are more often affected by needle stick injuries, post-contact HCV evaluation is essential (11).
The hepatitis A virus (HAV) mostly presents with acute hepatitis. It is mainly seen in childhood but may affect adults as well. However, over 90% of Turkey’s population is known to be immune to the virus (6). Due to the improvement in hygienic conditions, no increase in infection in the adult population has been observed (12). Again, from the perspective of HWs, it is possible to transmit the virus to susceptible personnel due to occupational risk. As the hepatitis B vaccine, the hepatitis A vaccine is one of the obligatory vaccines required by both the Centers for Disease Control and Prevention and the Ministry of Health (13).
During 1985 - 2013, 58 documented and 150 possible cases of occupational human immunodeficiency virus (HIV) transmission were reported in US health-care workers, implying a mean of five cases per year (14). Percutaneous puncture or cutting is defined as the most common mode of transmission (14). There is no seropositivity data on HIV in HWs seroprevalence studies in Turkey (5, 15-17).
Several studies have explored the seroprevalence of viral hepatitis and HIV in health professionals in the Republic of Turkey (18-22). However, these studies were not multiregional, thus reflecting the characteristics of a single institution and the province/geographical area where the institution is located (18, 19).
2. Objectives
This study aimed to determine HBV, HCV, and HIV seroprevalence among HWs using data obtained from 21 hospitals located in six geographical regions in Turkey and the Turkish Republic of Northern Cyprus (TRNC).
3. Methods
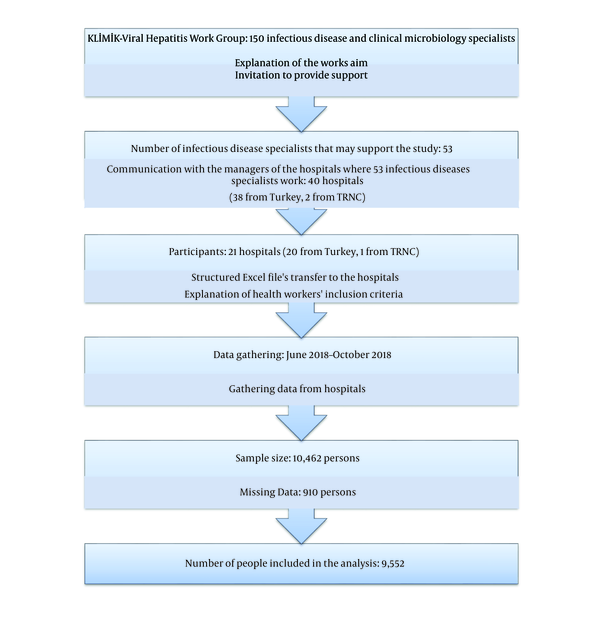
This study was designed as a retrospective, multicentre, descriptive study. A purposive sampling technique was adopted in this study, rather than a random sampling technique. The study was performed as a study of the Viral Hepatitis Working Group, a working group of the Turkish Society of Clinical Microbiology and Infectious Diseases. We explained the aim of the study to the infectious disease specialists in this study group. Then, they were asked if they could participate in this study. Contact was made with executives in charge of the 40 hospitals where the specialists work and to ask for their support (38 from Turkey and two from the TRNC). A total of 21 hospitals (20 from Turkey and the 1 TRNC) stated they could enroll in this study. The workflow of the study and the number and geographical distribution of the hospitals participating in the study are shown in Figures 1 and 2, respectively.
The study variables were vaccination status (i.e., having received at least one vaccine dose) against HBV and HAV and HBsAg (hepatitis B surface antigen), anti-HBs, anti-HBc IgG, anti-HAV IgG, anti-HCV, anti-HDV (hepatitis D virus) and anti-HIV serologic test results for the period of 2017 - 2018. Also, the following basic variables were included in the study: geographical region, hospital grade, age, gender, occupational duration, job, and department. For cases with HBsAg-positive results, HBeAg, anti-HBe, and HBV DNA level, treatment status and duration and agent(s) used for treatment were requested. For cases with anti-HCV-positive results, HCV RNA level, treatment status, and duration and agent(s) used for treatment were noted. For cases with anti-HDV positive results, HDV RNA level, and treatment status were obtained. Besides, where the anti-HIV test was positive, the HIV RNA level, antivirals used, and duration of treatment were requested. The criterion for the inclusion of the HWs in the study was having at least one HBsAg and anti-HBs laboratory result from the previous year. The exclusion criterion of the study was missing data for HBsAg and anti-HBs parameters in the prior year.
Health workers whose data in any of the mentioned variables were missing were also excluded from the analysis. Data collection was documented using a structured Excel file and lasted for four months (June 2018 - October 2018). Data from hospitals were gathered in a single file and checked. Hence, 910 persons were removed from the database, and the remaining 9,552 persons were analysed using the SPSS version 21.0. The descriptive findings of the study were presented as numbers and percentage distributions, means, standard deviations, and minimum and maximum values. P value of less than 0.05 was considered as the level of significance for all the statistical analyses, and the relationships were evaluated within the 95% confidence interval.
Ethics Committee approval for the study was obtained from Batman Regional State Hospital Ethics Committee for Non-Interventional Research (date: 13.06, 2018/number: 113). This article follows the tenets of the Declaration of Helsinki. Besides, permission was obtained from the management of each institution included in the study to allow the researchers to use the data.
4. Results
Data belonging to 9,552 HWs from 21 institutions were included in the study. In the geographical distribution of the HWs, the Central Anatolia Region ranked first with 32.9% (n = 3,140), while the Eastern Anatolia Region ranked second with 27.9% (n = 2,662). The Marmara Region was third with 17.7% (n = 1,695; Table 1). Six (28.5%) of the hospitals were secondary health-care facilities, while the remaining 15 were tertiary health-care hospitals. No private hospital was involved in this study.
| Variable | Values |
|---|---|
| Geographical Region | |
| Marmara Region | 1,695 (17.7) |
| Central Anatolia Region | 3,140 (32.9) |
| Aegean Region | 1,142 (12.0) |
| Mediterranean Region | 282 (3.0) |
| Eastern Anatolia Region | 184 (1.9) |
| South-Eastern Anatolia | 2,662 (27.9) |
| TRNCb | 447 (4.7) |
| Gender | |
| Female | 5,622 (58.9) |
| Male | 3,930 (41.1) |
| Occupation | |
| Physician | 2,365 (24.8) |
| Nurse/health officer | 4,064 (42.5) |
| Anesthesia technician | 341 (3.6) |
| Lab technician | 130 (1.4) |
| Paramedic | 9 (0.1) |
| Sanitation worker | 1,220 (12.8) |
| Department | |
| Administrative Employee | 1,057 (11.1) |
| Other Health Technicians | 2,17 (2.2) |
| Patient-Care Nurse | 149 (1.6) |
| Internal Service/Polyclinic | 3,374 (35.3) |
| Surgical Service/Polyclinic | 1,889 (19.8) |
| Administrative | |
| Intensive Care Unit | 1,107 (11.6) |
| Emergency | 691 (7.2) |
| Dialysis Unit | 207 (2.2) |
| Endoscopy/Colonoscopy | 87 (0.9) |
| Operating Room | 798 (8.4) |
| Laboratory | 716 (7.5) |
| Affairs | 297 (3.1) |
| Unknown | 386 (4.0) |
| Total | 9,552 (100.0) |
Descriptive Findings of the Study Groupa
Women constituted 58.9% (n = 5,622) of the HWs included in the study. The mean age was 36.3 ± 9.09 years (minimum = 18, maximum = 72). In terms of occupation, 42.5% (n = 4,064) of the HWs were nurses/health officers, 24.8% (n = 2,365) were physicians, 12.8% (n = 1,220) were sanitation personnel and 11.1% (n = 1057) were administrative officers. The mean occupation duration of the participants was 12.33 ± 9.05 years (minimum = 1 year, maximum = 51 years). About 35.3% (n = 3,374) of the participants were internal service/polyclinic staff members, 19.8% (n = 1,889) were surgical service/polyclinic staff members and 11.6% (n = 1107) were staff members of the intensive care unit (Table 1).
HBsAg seroprevalence was found to be 1.8% (n = 169; 95% CI = 1.5% - 2.0%), and anti-HBs seroprevalence was 75.7% (n = 7,234). About 7.3% (n = 701) had natural immunity to hepatitis B. About 21.6% (n = 2,066) of the HWs had not received the hepatitis B vaccine. When those with natural immunity to hepatitis B were excluded, 17.0% (n = 1,502) of the remaining 8,851 HWs had not received the hepatitis B vaccine. All the HWs who took at least one dose of the hepatitis B vaccine were positive for anti-HBs. Among those who were HBsAg-positive (n = 169), 5.3% of the workers were also HBeAg-positive, 81.1% were anti-HBe-positive, and 79.3% were anti-HBc IgG positive. Of the HBsAg-positive workers, 26% (n = 44) were receiving treatment. The mean HBV DNA level was found to be 111556.83 ± 598561.41 IU/mL (minimum = 0, maximum = 4980000). In 29.6% of the HBsAg-positive workers, HBV DNA level was over 200 IU/mL, but in 17.2% of them, it was above 2000 IU/mL. Among those receiving antiviral treatment, 50% of the HWs were taking tenofovir disoproxil fumarate, while 22.7% were taking entecavir. The mean duration of treatment was 123.52 ± 98.72 weeks (minimum = 8, maximum = 340; Table 2).
| Variable | Values |
|---|---|
| HBsAg | |
| Positive | 169 (1.8) |
| Negative | 9,383 (98.2) |
| Anti-HBs | |
| Positive | 7,234 (75.7) |
| Negative | 2,318 (24.3) |
| Hepatitis B vaccine | |
| Vaccinated | 4,581 (48.0) |
| Unvaccinated | 2,066 (21.6) |
| Unknown | 2,905 (30.4) |
| Hepatitis B natural immunity | |
| Yes | 701 (7.3) |
| No | 6,406 (67.1) |
| Unknown | 2,445 (25.6) |
| Anti-HAV IgG | |
| Positive | 3,838 (40.2) |
| Negative | 1,070 (11.2) |
| Unchecked | 4,644 (48.6) |
| Anti-HCV | |
| Positive | 32 (0.3) |
| Negative | 9,463 (99.1) |
| Unknown | 57 (0.6) |
| Anti-HIV | |
| Positive | 1 (0.01) |
| Negative | 9,440 (98.8) |
| Unknown | 111 (1.2) |
| Total | 9,552 (100.0) |
HBV, HAV, HCV and HIV Seroprevalence of the Study Groupa
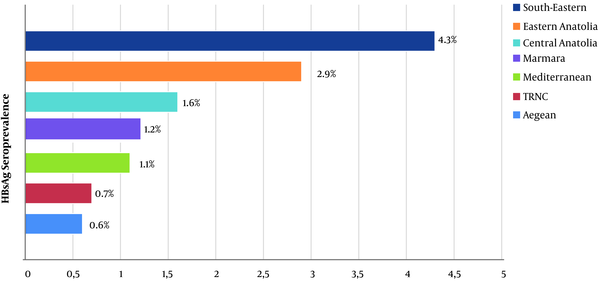
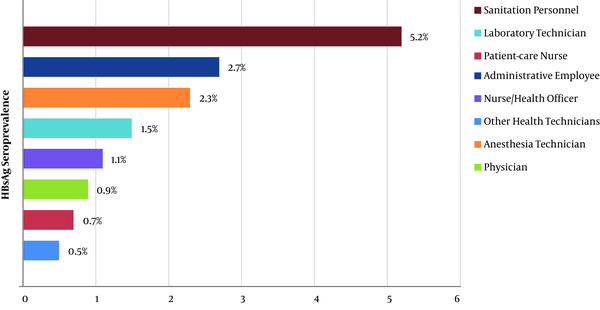
HBsAg seroprevalence among the male HWs (3%) was significantly higher than among the female workers (0.9%; P < 0.001). When anti-HBs seroprevalence data was examined, it was found to be significantly higher in women than in men (81.7% vs. 41.1%; P < 0.001). According to geographical regions, for HBsAg positivity, the South-Eastern Anatolia Region had the highest seroprevalence (4.3%), while the Eastern Anatolia Region ranked second (2.9%) (P < 0.001; Figure 3). The occupational group with the highest HBsAg seroprevalence was the sanitation personnel (5.2%). The administrative officers ranked second (2.7%), and the anaesthesia technicians ranked third (2.3%). HBsAg was found to be positive in 0.9% of the physicians, 1.1% of the nurses/health officers, and 1.5% of the laboratory workers (P < 0.001; Figure 4). The occupational groups with the highest anti-HBs seroprevalence were laboratory technicians (93.1%), physicians (91.5%), anaesthesia technicians (91.2%), and other health technicians (89.3%). Anti-HBs was positive in 77.6% of the nurses/health officers and 52.6% of the sanitation personnel (P < 0.001). When compared according to the department, HBsAg seroprevalence was highest among endoscopy/colonoscopy workers (3.4%). This was followed by laboratory workers (2.2%), internal service/polyclinic workers (1.8%), intensive care unit workers (1.7%), emergency department workers (1.6%), and surgical service/polyclinic workers (1.5%) (P < 0.001). In terms of anti-HBs seropositivity, the operating room workers had the highest percentage (82.6%). This was followed by the dialysis unit workers (81.6%), internal service/polyclinic workers (80.1%), and laboratory workers (79.7%) (P < 0.001; Table 3).
| Variable | HBsAg | P Valueb | |
|---|---|---|---|
| Negative | Positive | ||
| Gender | < 0.001 | ||
| Female | 5,572 (99.1) | 50 (0.9) | |
| Male | 3,811 (97.0) | 119 (3.0) | |
| Geographical regions | < 0.001 | ||
| South-Eastern Anatolia | 176 (95.7) | 8 (4.3) | |
| Eastern Anatolia | 2,585 (97.1) | 77 (2.9) | |
| Central Anatolia | 3,089 (98.4) | 51 (1.6) | |
| Marmara | 1,675 (98.8) | 20 (1.2) | |
| Mediterranean | 279 (98.9) | 3 (1.1) | |
| TRNCc | 444 (99.3) | 3 (0.7) | |
| Aegean | 1,135 (99.4) | 7 (0.6) | |
| Occupation | < 0.001 | ||
| Sanitation personnel | 1,156 (94.8) | 64 (5.2) | |
| Administrative employee | 1,028 (97.3) | 29 (2.7) | |
| Anesthesia technician | 336 (97.7) | 8 (2.3) | |
| Laboratory technician | 128 (98.5) | 2 (1.5) | |
| Nurse/health officer | 4,021 (98.9) | 43 (1.1) | |
| Physician | 2,344 (99.1) | 21 (0.9) | |
| Patient-care nurse | 148 (99.3) | 1 (0.7) | |
| Other health technicians | 213 (99.5) | 1 (0.5) | |
| Paramedic | 9 (100.0) | 0 (0.0) | |
| Age, y | 0.002 | ||
| 18 - 30 | 2,950 (98.9) | 34 (1.1) | |
| 31 - 40 | 3,621 (98.1) | 72 (1.9) | |
| 41 - 50 | 2,077 (97.5) | 54 (2.5) | |
| 51 - 60 | 642 (98.9) | 7 (1.1) | |
| > 60 | 93 (97.9) | 2 (2.1) | |
| Department | < 0.001 | ||
| Endoscopy/ Colonoscopy | 84 (96.6) | 3 (3.4) | |
| Laboratory | 700 (97.8) | 16 (2.2) | |
| Internal Service/Polyclinic | 3,312 (98.2) | 62 (1.8) | |
| Intensive care unit | 1,088 (98.3) | 19 (1.7) | |
| Emergency | 680 (98.4) | 11 (1.6) | |
| Surgical Service/Polyclinic | 1,861 (98.5) | 28 (1.5) | |
| Dialysis Unit | 204 (98.6) | 3 (1.4) | |
| Administrative Affairs | 293 (98.7) | 4 (1.3) | |
| Operating Room | 792 (99.2) | 6 (0.8) | |
| Unknown | 369 (95.8) | 17 (4.4) | |
| Total | 9,383 (98.2) | 169 (1.8) | |
Variables Associated with HBsAg Seroprevalencea
Anti-HAV IgG was positive in 40.2% of the HWs. About 3.1% of those with anti-HAV IgG negative results had received the hepatitis A vaccine. Anti-HCV seroprevalence was 0.3% (n = 32). Anti-HDV positivity was not detected among the HWs, and anti-HIV seroprevalence was less than 0.01% (n = 1).
5. Discussion
HBV infection is a well-recognized occupational risk for health-care workers (23). In this context, the Republic of Turkey's Ministry of Health has been conducting regular screenings of health-care workers for hepatitis B, hepatitis C, and HIV since 1996 (24). Vaccination of susceptible health professionals is performed under the Hepatitis B Prevention Program and the Extended Program on Immunization (7).
Previous studies conducted in the Republic of Turkey have revealed that the seroprevalence of HBsAg among health professionals varies between 0.2% and 2% (6). In the present study, this rate was found to be 1.8%. However, HBV prevalence among health professionals was significantly lower than the overall prevalence in Turkey (25). In contrast to other studies, the seroprevalence of the disease in the South-Eastern Anatolia Region was found to be higher than in all the other geographical regions. This finding could be the reflection of overall HBV prevalence in the region (4).
Furthermore, the prevalence of HBV in blood donors was found to be 2.3% in the TRNC. The seroprevalence of HBV was at the lower limit of the average of Turkey and near those of developed countries (26). Similarly, in this study, the seroprevalence in the TRNC was at the lower limit of the average of Turkey.
The rate of HBV infection in males was found to be significantly higher than in females. The percentage of HBV infection was much higher among sanitation personnel, compared to the other HW groups. Similarly, HBV seroprevalence was found to be higher, and the hepatitis B vaccination rate was found to be lower in sanitation staff in the literature (27-30). It is thought that the sanitation staff members may have experienced hepatitis B before starting the profession because of their lower socioeconomic levels, compared to the other groups. Also, it is known that the majority of high-risk contacts occur with medical waste (28). To reduce the frequency of such contacts, it is considered that health personnel, including sanitation personnel, should be trained on the proper separation of medical waste (27-30). Among sanitation personnel, hepatitis B vaccination should be increased because of the high risk of hepatitis B transmission and the low incidence of protective antibodies. These results are in accordance with the epidemiological distribution of HBV in the Republic of Turkey (27-30). The seroprevalence of anti-HBs was higher among anaesthesia technicians and doctors than the other groups. The seroprevalence of anti-HBs in this study was higher than in previous studies (5, 21, 31, 32).
When anti-HBs positivity rates were examined in similar studies in the Republic of Turkey, it was found that the rate of positivity increased from about 50% in the 1990s to about 65% in the next decade. In a study by Korkmaz et al. (5), this rate was found to be 86%. HBV screening and vaccination in HWs are considered the cause of this increment (33). In our study, anti-HBs seropositivity was found to be 75.7%. Although the proportion of people with immunity for hepatitis B was high, the percentage of HWs susceptible to the disease was calculated to be approximately 20%, excluding administrative officers. One out of five HWs was at risk of the disease. This result suggests vaccination is still not comprehensive enough among HWs and reveals the importance of follow-up and vaccination. In this study, all the HWs who had received at least one dose of the hepatitis B vaccine were positive for anti-HBs. Thus, the three-dose Hep B vaccine series could produce a protective antibody response (2). Endoscopy has been associated with the risk of HBV transmission, as indicated in some previous studies (34, 35), but in our research, we did not find increased ratios in Endoscopy units.
A group of HWs infected with hepatitis B who were untreated and had high viral loads were identified in this study (n = 29). Chronic hepatitis B does not prevent health professionals from working in any department, including Surgical departments. In line with the European Association for the Study of the Liver’s recommendations in 2017, HBV DNA levels should be below 200 IU/mL for HWs. In light of this recommendation and our findings, there is a group of HWs in our country that should receive antiviral treatment (36).
The rate of anti-HCV positivity was found to be less than 0.5% in almost all the previous studies conducted in Turkey (19). Similarly, it was found to be 0.3% in our study. Individuals with HCV RNA positive test results need to be treated with oral antiviral regimens, which are now quite simple and effective.
The rate of anti-HAV IgG seropositivity in the Republic of Turkey has been found to vary in different studies. In this study, 11.2% negativity was found among the participants that could be examined for anti-HAV IgG (only half of the participants included in the study had anti-HAV IgG results). The Republic of Turkey is a country with moderate endemicity in terms of hepatitis A, and it is essential to screen the high-risk group and vaccinate susceptible persons, considering hepatitis A is much more severe in adulthood. In addition, the WHO recommends vaccinating all susceptible persons (37, 38).
To the best of our knowledge, HIV-infected health professionals have never been reported in other studies from Turkey, but one health professional with HIV was identified in our study, and we have no information about whether this case was exposed to HIV in the health care setting or not. This finding suggests a higher prevalence of HIV infections may exist in the future (5, 21, 31).
Even though this study involved a large sample size, there are some limitations. First, the HWs’ blood-borne disease risk factors were not examined in detail. Second, the data obtained from the hospitals were based on the data of the HWs in the previous year, excluding HWs without data for that year. Third, the hospitals that thought there was a lack of data in terms of the variables collected in the study may not have agreed to participate in the study. If these hospitals had participated in this study, we might have obtained different results. Fourth, generalization of the findings, considering the sampling methodology, to all health-care workers of all hospitals located in Turkey and the TRNC may not be appropriate. Within the Expanded Program on Immunization, hepatitis B vaccination of all health-care workers in Turkey is planned, and the relevant serological tests are examined before vaccination. In addition, in accordance with Occupational Health and Safety Law no.: 6331, serological tests are to be carried out at regular intervals. For that reason, even if a workgroup had been selected using a sampling method that represents all hospitals in Turkey and the TRNC, similar results would have been expected.
5.1. Conclusions
This study is the first with a large sample size from different locations in the Republic of Turkey. According to the results of this study, hepatitis B and hepatitis A vaccines should be administered to susceptible individuals and HWs. In particular, sanitation personnel, who have a low rate of anti-HBs positivity and come in contact with medical waste, should be offered hepatitis B vaccination once they are employed. Follow-up should also be performed to identify infected workers and to provide them appropriate treatment. If this is done, the Republic of Turkey can achieve the goal of eliminating viral hepatitis by 2030, which is a global target.