1. Background
An anti - neoplastic drug can lead to cell death with different modes. Generally, apoptosis induction is the most reaction of the damaged cells by chemotherapeutic agents. Necrosis is another death mode that occurs as a passive process (1). The nature of stimulus or cell death signal, cell type, and developmental phase of a cell are factors that run into a cell to apoptosis or necrosis occurrence (1,2). In addition, arresting in one of the phases of the cancer cell cycle could be induced by some drugs (2).
The anthracyclines such as daunorubicin (DNR) is capable of killing the human acute myeloid leukemia (AML) cells through apoptosis or necrosis with arresting cell cycle and various mechanisms (3-5). The relatively mature human acute myeloid leukemia (AML) cell line U937 shows more sensitivity to DNR than the immature ones within concentration range 0.5 μM to 2 μM (6,7). The response of AML cells to DNR associated with defect and or defiance in survival has been a consideration subject. So far, some mediators have been suggested in DNR action, including the induction of the free radical production, the activation of the PI3K/AKT pathway, and the NF - kB stimulation (3).
These reports of DNR effects on U937 cells led us to investigate the killing way and cell cycle ceasing manner of DNR in U937 cells. Meanwhile, we have represented the transcription gene profile of some critical prosurvival proteins by qRT - PCR, including osteopontin (OPN), AKT1, mTOR, β - catenin, and NF - kB/RelB.
2. Methods
2.1. Materials
Danarubicin (Pharmacia & Upjohn SpA; Milan, Italy) was dissolved in distilled water to prepare 1 mg/mL stock solution and 100 µg/mL work solution immediately before use. Annexin V - Alexa Fluor - 488/PI kit was purchased from BD Biosciences (San Jose, CA, USA). The human monoclonal anti - bodies, such as PE anti - CD34, FITC, and anti - CD38 were purchased from BD Biosciences (San Jose, CA, USA). Tripure isolation Reagent was purchased from Roche Applied Science (Germany). The cDNA synthesis kit and SYBR®Premix Ex Taq™were purchased from Takara Biotechnology Co. (Otsu, Japan).
2.2. Cell Culture
The human leukemic monoblast U937 cell line obtained from the Pasteur Institute of Iran. RPMI 1640 - 10% FBS mediums (Gibco; Invitrogen, USA) were used for culturing U937 cell line. This medium was supplemented with 2 mM L - glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
2.3. MTT Assays
Cells were cultured in triplicated at 5 × 103/100 µL in 96 - well culture plates (SPL Life sciences, Pocheon, Korea) with clinically achievable concentrations 0.5, 1, 2 μg/mL of danarubicin for 20 to 24 hours. The cells were incubated (37 °C , 5% CO2) for 4 hours with 3 - (4,5 - dimethylthiazol - 2 - yl) - 2,5 - diphenyltetrazolium bromide (MTT, 5 mg dissolved in 1mL of PBS, Sigma, St. Louis,MO, USA). The plates were spun, and the purple formazan crystals of metabolized yellow tetrazolium salt by viable cells were dissolved in DMSO. Absorbance was quantified at 570 nm, using the ELISA plate reader (Micro plate Reader; Bio - Rad). Results were expressed as a percentage of viability, with 100% representing control cells treated with 0.1% DMSO alone.
2.4. Evaluation of Apoptosis by Annexin V/Propidium Iodide (PI) Assay
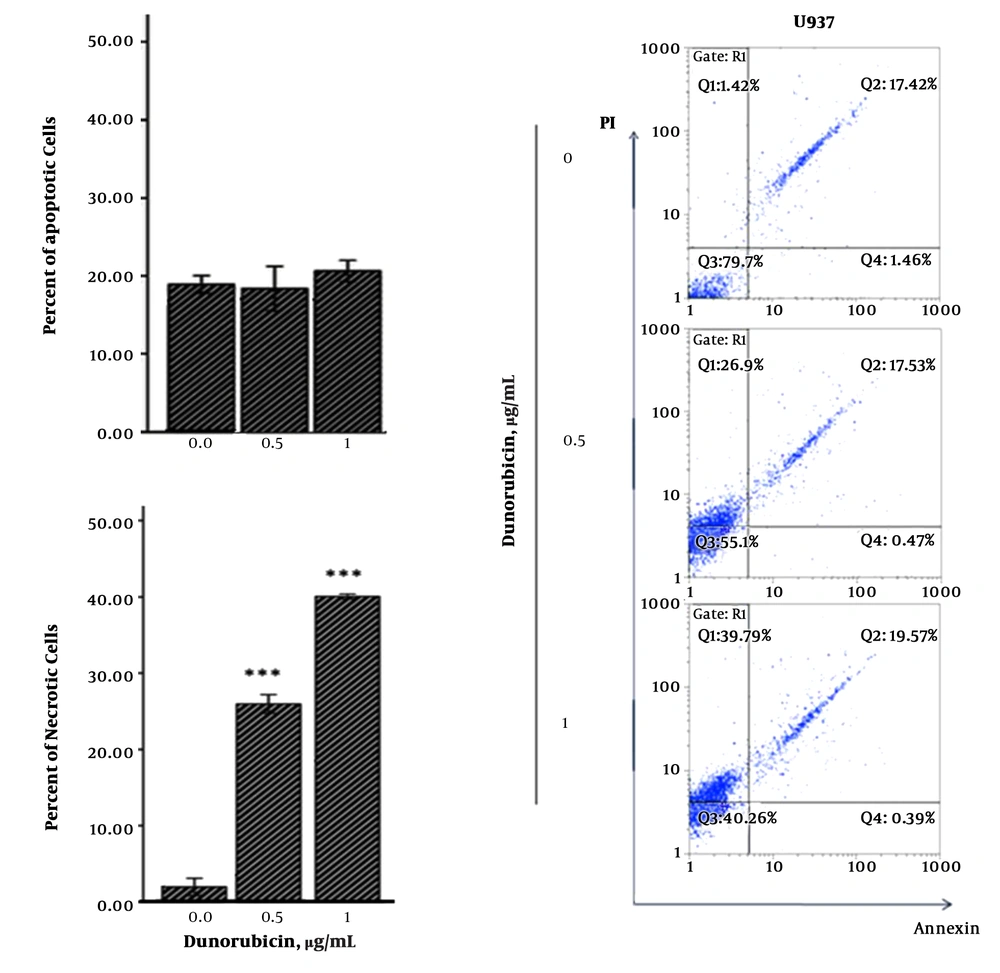
A density of 1 × 106/mL cells per well in 6 - well plates was treated with DNR in indicated concentrations. After 24 hours, cells were harvested and mixed with Annexin V - Alexa Fluor - 488/PI according to the manufacturer’s instruction. The stained cells were examined by flow cytometry (Partec, Munich, Germany). Discrimination of cells was performed as apoptosis (Annexin V+/PI- [early apoptosis] and Annexin V+/PI+ [late apoptosis]) and necrosis (Annexin V-/PI+).
2.5. Cell Cycle Analysis
Approximately, 106cells/mL were treated with increasing concentrations of DNR 0.5, 1, and 2 μg/mL at 24 hours. The cells staining with propidium iodide (Sigma - Aldrich, Spain) was performed, using manufacturer’s instruction. Cell cycle was analyzed by flow cytometry.
2.6. Quantitative Real - time PCR (qRT - PCR)
Total RNA of the DNR - treated and untreated cell lines were extracted with Tripure isolation Reagent according to the manufacturer’s instruction, qauntited, using drop ND - 1000 (Nano drop Technologies, Wilmington, DE), and stored at - 80°C. The cDNA synthesis kit was used for complementary DNA (cDNA) synthesis. A light cycler instrument (Roche Diagnostic, Manheim, Germany) and SYBR Premix Ex Taq were used for Quantitative Real-time RT - PCR analysis. A final volume of 20 µL containing 2 µL of a 2 - fold diluted cDNA, 1 µL of 10 pmol primers (0.5 µL each forward and reverse primers), 10 µL of SYBER, and 7 µL distilled water were used. The data were normalized to HPRT expression in each sample. The analysis of relative gene expression data was performed, using the 2- ∆∆Ctmethod. We determined the efficiency of the quantitative PCR assay, by means of standard curves. Table 1 shows the primer sequences for genes used.
| Gene | Forward Primer (5' - 3') | Reverse Primer (5' - 3') | Size (bp) |
|---|---|---|---|
| HPRT | TGGACAGGACTGAACGTCTTG | CCAGCAGGTCAGCAAAGAATTTA | 111 |
| OPN | ACCCTTCCAAGTAAGTCCAACG | GGTGAGAATCATCAGTGTCATCTAC | 139 |
| NF - B/RelB | GCCTGACTTTGAGGGACTGTA | CTAGATGCAAGGCTGTTCGTC | 137 |
| AKT1 | AGCGACGTGGCTATTGTGAAG | GTACTCCCCTCGTTTGTGCAG | 51 |
| mTOR | AACTCCGAGAGATGAGTCAAGA | AGTTGGTCATAGAAGCGAGTAGA | 49 |
| PTEN | TGGATTCGACTTAGACTTGACCT | TTTGGCGGTGTCATAATGTCTT | 184 |
| β - Catenin | TACCTCCCAAGTCCTGTATGAG | TGAGCAGCATCAAACTGTGTAG | 180 |
2.7. Flow Cytometry
Cell lines were treated with DNR at 24 hours. Before labeling, the cells were spun to remove the debris and re - suspended in PBS. Then, the cells were stained with the panel of human monoclonal anti - bodies, including PE anti - CD34, FITC, and anti - CD38. An appropriate isotype control IgG1 was used. The analysis was carried out by a Partec PAS III flow cytometer (Partec, Munich, Germany), and the data were interpreted, using the FloMax software.
2.8. Statistical Analysis
Using IBM SPSS Statistics 19 software, the groups of data were presented as means ± SD and compared by one - way analysis variance (ANOVA).
3. Results
3.1. DNR Induced Death Through Necrosis in Mature Cell Line U937
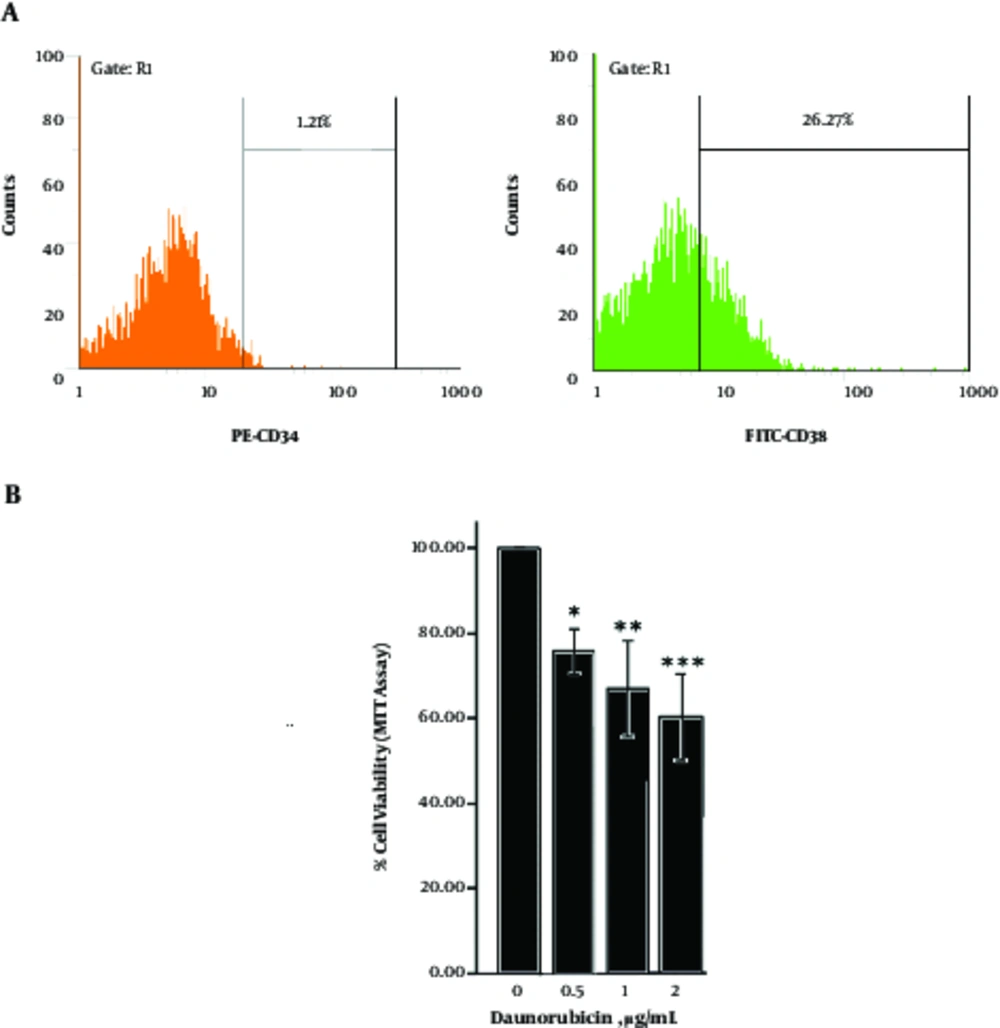
According to immunophenotypically common maturation - associated profile, the mature CD34 - CD38+ U937 cells exhibited sensitivity to different concentrations of DNR by MTT - based cytotoxicity assays at 24 hours (Figure 1 A, B). The Annexin V/PI assay displayed that DNR - induced death in cells was a dose - dependent and necrotic manner (Figure 2).
(A) Flow cytomeric analysis of U937 cell line stained with monoclonal anti - body PE - CD34 and FITC - CD38 showed maturation - associated profile the mature CD34- CD38+. (B) Dose - response curves with different concentrations of DNR, using MTT assay/24 hours exhibited sensitivity to different concentrations of DNR. The graphs represent 3 independent experiments (mean ± SD). *P < 0.05, **P < 0.01, ***P < 0.001 (compared with control or comparisons depicted).
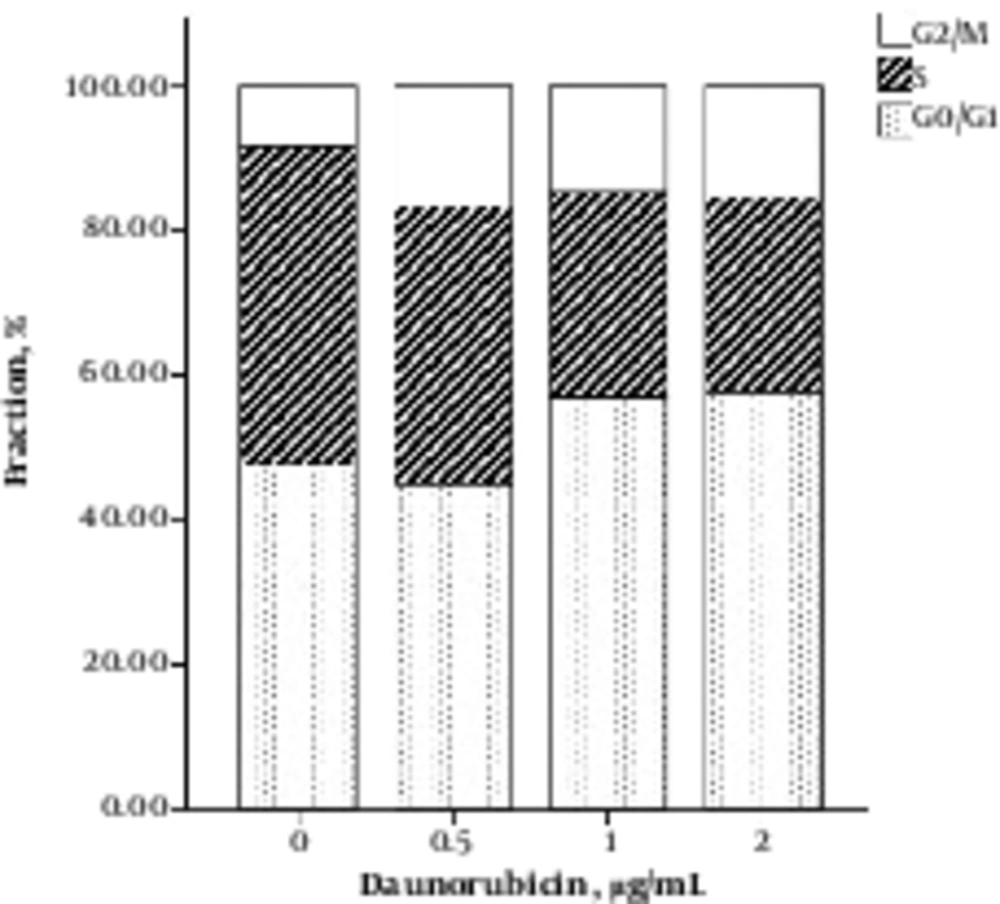
Cell cycle distribution following treatment with DNR exhibited a relatively lower chromatin of S phase than untreated cells (Figure 3). This finding corresponds to the DNA stainability pattern of necrotic cells that does not distinguish them from live cells (8).
3.2. DNR Decreased the mRNA Expression of OPN, but not NF - kB/RelB, AKT1, mTOR, β - catenin, or PTEN
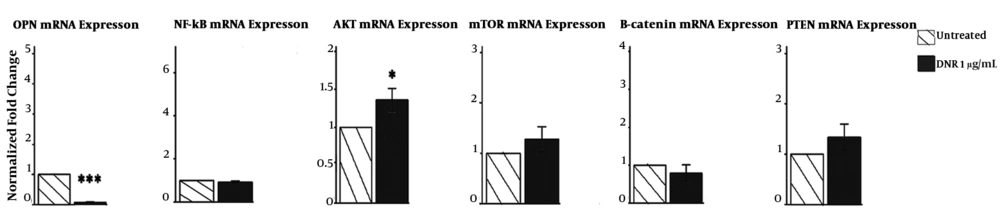
We investigated the effects of DNR on transcriptional profile of some putative cytoprotective mediators in response to chemothrapy, that is, the mRNA levels of OPN, NF - kB/RelB, AKT1, mTOR, β - catenin, as well as PTENby quantitative RT - PCR. Despite the other genes, OPNgene expression was significantly attenuated. NF - kB/RelB, mTOR, β - catenin, as well as PTENgenes showed unchanged or non - significant increase in expression. However, AKT1increased significantly (Figure 4).
The transcriptional profile by qrt - PCR presented that DNR decreases the mRNA expression of OPN, but not NF - kB/RelB, AKT1, mTOR, β - catenin, or Pten. The graphs represent 3 independent experiments (mean ± SD). *P < 0.05, **P < 0.01, ***P < 0.001 (compared with control or comparisons depicted).
4. Discussion
In the present research, we found that DNR can induce a necrotic death in U937 cell line. Although U937 cell is known as a DNR-sensitive (6), to the best of our knowledge, there is no report of necrotic death induction of DNR in these cells. Interference with the energy supply of the cell and direct damage to cell membranes are the main features of necrosis (1). Since, on one hand, DNR brings on reactive oxygen species (ROS) (9), and, on the other hand, excessive ROS brings about necrosis by depletion of intracellular ATP (10), one could hypothesize that ROS could be involved in DNR - induced necrosis. Besides, the DNR distribution in U937 and other CD34-cells is independent of energy with nuclear and diffuse cytoplasmic pattern. This DNR sequestration has been suggested that interfere with cytotoxicity (11). Generally, the mode of cell death by anthracyclines could be different as a dependent on concentrations and duration of the inducement. Evaluating the human leukemia cell lines HL - 60 and jurkat T cells shows that DNR or doxorubicin conducts to cell membrane disruption abruptly or gradually (4,5). By contrast, DNR - induced apoptosis in U937 cells has been described (7).
When a cell is sensitive to an agent, this means that it has failed to intercept toxic effects of drug with defense mechanisms, as we have observed to occur in the pattern of the transcription of some crucial prosurvival genes. Despite the other studied genes, decrease in OPNgene transcription was so significant that it suggests a prominent role of an impressed OPNin sensitization of U937 cells to DNR. As a constituent part of extracellular matrix in bone marrow (BM), OPN regulates haemopoietic stem cell (HSC) pool size. While a HSC differentiates, OPN inhibition effects on its proliferation reduce (12). Also, interaction between acute lymphoblastic leukemia (ALL) cells with OPN in BM inhibits cell cycle to save them from killing the effect of Ara - C chemotherapy (13). In other reports, OPN is able to mobilize CD34+cells (14) or positively regulates haemopoietic cells survival (15). High OPN levels have association with the progression of chronic and acute myeloid leukemia as well as multiple myeloma cell survival (12,16,17). Additionally, in solid tumors, the increased expression of OPN mRNA has promoted proliferation (18,19). In this regard, U937 cells may susceptible to death through DNR - reduced OPN gene expression.
We found a slight reduction in NF - kBand β - cateningene expression with DNR treatment. NF - kB and β - catenin result in the development of human cancer through involving in the regulation of apoptosis and proliferation as well as chemo-resistance (20-22). Previous studies have demonstrated that the amount of β - catenin is increased in transfected U937 cells with AML - associated translocation products; in turn, it is associated with an increase in cell cycle progressing proteins, c - Myc, and cycline D1 (23). Thus, lowering their gene transcription by DNR could, in part, be contributed in failed survive of U937 cells. Besides, NF - kB activation by DNR stimulation has been reported (24).
As cytoprotective activation, U937 cells have tried to increase the AKTand mTORgene transcription in response to DNR. The function of the PI3K pathway, including various downstream mediators, such as AKT and mTOR plays an important role in protective against apoptosis induction of chemotherapy (25). In a study, it was shown that DNR stimulates an increase in PI3K/AKT activity in U937 cells (26). Given that AKT is the only effector that significantly has increased, one can speculate that it could not be sufficient to save cell from DNR harm. Increasing trend in PTEN expression might occur simultaneously with AKT or mTOR (27). Even though it has been reported that there is an abnormal transcript of PTEN in U937 cells, they try to increase the amount of PTEN to help cause an apoptosis (28).
4.1. Conclusions
One possible explanation of U937 sensitivity to DNR could be the targeting of anti - apoptotic proteins in the transcriptional stages. In this context, increase in the expression of some important anti - apoptotic genes, including AKT1, mTOR, NF - kB, and β - cateninand vice versa reduction in OPNlevels suggest that the OPN might play an imperative role in preventing the death observed by DNR.