1. Context
Tachykinins (TKs) are an evolutionarily conserved family of peptide hormones, which are widely distributed within the peripheral and central nervous systems. Various types of TKs are produced from more significant precursors, named preprotachykinins (1). The tachykinin peptide family shares the typical carboxyl-terminal sequence, -Phe-X-Gly-Leu-Met-NH2, where X is a variable amino acid residue (2, 3). Several lines of evidence showed that the TAC1 gene produces substance P (SP) and neurokinin A (NKA), which are the most critical TKs family. Additionally, NKB and NKF are encoded by the TAC3 gene, and the TAC4 gene encodes two other important TKs named hemokinin-1 and different kinds of endokinins (EK-A to EK-D) in various tissues, including heart, liver, and placenta (4, 5).
TKs exert their biological functions through binding to three subtypes of transmembrane G-protein coupled receptors (NK1R, NK2R, and NK3R) in many processes (6). Previously, neurons had been detected as the critical source of TKs. Nowadays, it is well-identified that TKs and their receptors contribute to the effective connections between the nervous system and respiratory, cardiovascular, immune, endocrine, gastrointestinal, and genitourinary systems (7-11). For instance, TKs in the respiratory tract have a vital role in moisturizing the airways (12). Also, TKs are involved in smooth muscle contraction of the ureter (13), as well as the contraction of uterus and abortion (14). Although the mechanisms that connect TKs peptide activity to physiological processes are currently precise, it has been shown that TKs over-activation is associated with the pathogenesis of many diseases, including pain, emesis, depression, stress, Parkinson’s disease and inflammatory processes in the respiratory system (15, 16). Additionally, several studies have indicated that the TKs overexpression may also contribute to the progression and development of various tumors, mainly gastrointestinal (GI) malignancies (Figure 1) (12, 17, 18).
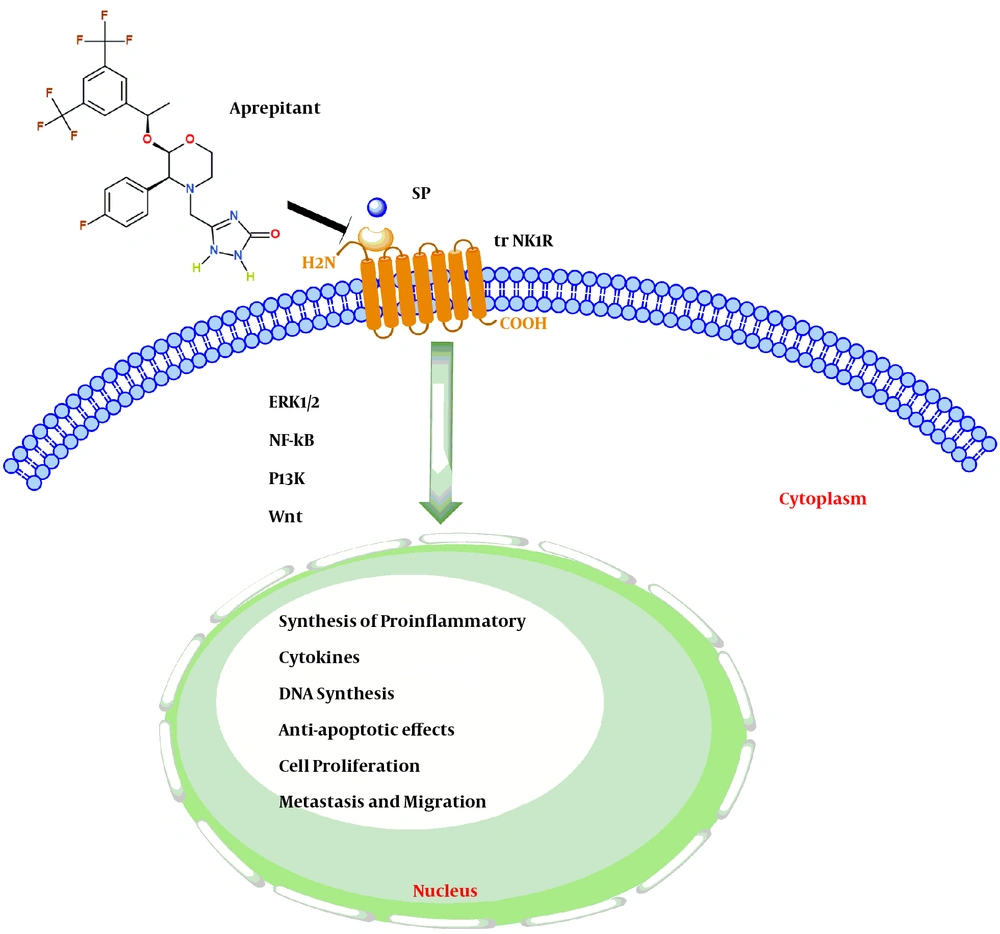
Schematic highlighting the SP-NK1R signaling pathways. Activation of the trNK1R by SP lead to induced cell proliferation, anti-apoptotic effects, Synthesis of proinflammatory cytokines, and migration of gastrointestinal cancer cells by activating several signaling pathways (ERK1/2-NFKB-PI3k-Wnt). While the use of Aprepitant, as an NK1R antagonist, also reduce or even inhibit the growth of gastrointestinal cancer cells.
GI cancers refer to malignant conditions of the GI tract, which are among the most prevalent diseases and are the leading cause of cancer-related death worldwide (19). Recent studies have shown that the binding of TKs to specific cellular receptors mediates a critical GI tumor proliferation pathway via initiation and activation of effector mechanisms, including protein synthesis and progression of the eukaryotic cell cycle (Figure 1) (20).
Hence in this review, we attempt to elucidate the critical role of TKs in initiation and progression of GI cancers including oral squamous cell carcinoma (OSCC), esophageal squamous cell carcinoma, gastric cancer, colorectal cancer, hepatocellular carcinoma, cholangiocarcinoma, and pancreatic cancer.
2. Evidence Acquisition
Review was conducted using keywords such as “Tachykinins”, “Gastrointestinal cancer”, “Metastasis”, “G-protein coupled receptors”, and “pharmacological inhibitor” thorough search in PubMed, Science Direct, Google Scholar, and Scopus websites without any time limit. The latest articles and books were reviewed. Articles and books which had constructive information to the topic were further comprised in the current study.
3. Results
3.1. TKs and Oral Squamous Cell Carcinoma
Oral squamous cell carcinoma (OSCC) is the most common and lethal malignant epithelial neoplasm that is affecting the upper and lower portions of the mouth (21). OSCC generally has a poor prognosis due to its tendency towards invasion and consequent metastasis (22). Recently, researchers have shown that TKs overexpression can lead to abnormal cellular growth, which underlies the process of OSCC tumorigenesis (23, 24). Additionally, experimental studies suggested a positive correlation between SP/NK1R overexpression and neutralization of apoptotic cell death in OSCC (24). Similarly, Mehboob et al. (25) showed that the SP expression is increased in highly advanced stages of malignancies (poorly differentiated). In contrast, its expression is decreased in the early stages of tumor cells (well-differentiated). Collectively, these results suggest that SP overexpression may play a critical role in an advanced stage of OSCC (25). Furthermore, in another study, Obata et al. (26, 27) evaluated the relationship between the TAC3 gene and OSCC pathogenesis. Accordingly, they found that the TAC3 gene was overexpressed in sensory neurons of the mandible and thereby involved in the development and progression of OSCC despite its significant role in the male reproductive system (26, 27). Taken together, these findings support the hypothesis that NK1R-targeted therapy can be considered as a potential therapeutic approach in some OSCCs.
3.2. TKs and Esophageal Squamous Cell Carcinoma
Esophageal squamous cell carcinoma (ESCC), as a heterogeneous malignancy, is one of the most prevalent and lethal forms of tumor worldwide and is the eighth most common malignancy globally (28, 29). Recently, researchers have been studying the mediator effects of TKs on both physiologic and pathologic conditions of the esophagus (30-33). Accordingly, Kovac et al. (34) revealed that the existence of TK-containing neurons in human esophageal smooth muscle cells (SMCs) can initiate the contraction through a series of mechanisms, including Ca2+ influx, Ca2+ release from stores, and the activation of non-selective cation current (INSC). Similarly, Zhang et al. (35) provided evidence that the TACR2α gene expression, which encodes a functional isoform of the NK2 receptor is upregulated compared to other isoforms. These results suggest that TKs via binding to NK2R may play a pivotal role in the contraction of the clasp and sling fibers of the lower esophageal sphincter complex (35).
Although in some experimental studies, TKs are involved in physiological processes, it has been suggested that these neuropeptides may play a significant role in initiating and triggering inflammatory responses, which thereby leads to increased cancer progression and development (36, 37). In line with this, Nascimento et al. (38) found that megaesophagus, as a result of the destruction of parasympathetic innervation, is associated with an imbalance between increased SP expression level and decreased vasoactive intestinal polypeptide. Consistent with these results, our previous experimental study showed that administration of aprepitant (15 µM), as a highly selective NK1R antagonist, exerts its potent antitumor effects against ESCC cells through the suppression of PI3K/Akt axis, and its downstream effector molecules including the activation of NF-κB and apoptosis evasion (39). Similarly, another study found that SP/NK1R overexpression has a positive correlation with tumor size and lymph node metastasis. These results support the idea that SP mediates pro-migratory responses via increased intracellular calcium levels in ESCC cells (40). Additionally, Wang et al. (41) observed that NK1R overexpression can enhance cell growth both in vitro and in vivo, representing its proliferative role in the progression and development of ESCC cells. Collectively, these data suggested that the significant role of NK1R in regulating ESCC cell growth depends on the SP overexpression and may be related to the downstream effectors (41).
It is well established that promoter hypermethylation is a critical way to silence the expression of tumor suppressor and tumor-related genes during tumorigenesis (42). The promoter hypermethylation is potentially reversible and can be considered as a main epigenetic modification in human cancers (43, 44). In line with this, the TAC1 hypermethylation has been frequently detected in a variety of human cancer cells, including head and neck cancers, which subsequently leads to repressed expression and function of the TAC1 gene (45). For instance, Jin et al. (46) demonstrated that the promoter hypermethylation of the TAC1 gene is associated with poor prognosis and can be served as an accurate predictor of ESCC progression and development.
3.3. TKs and Gastric Cancer
Gastric cancer (GC) is the fourth most common type of cancer and the second cause of cancer-related death in the world (47). GC remains a significant health problem because there is no reliable marker that could be used for the early-stage diagnosis (48). Hence, the development of new treatment and diagnostic strategies is required. Accordingly, several studies have investigated the correlation between TKs expression and GC progression (49, 50). For instance, Munoz et al. (50) showed that the SP nuclear expression in GC cells is significantly higher than compared to healthy controls, and thereby suggested that SP overexpression may be considered as an epigenetic factor, which can regulate the GC cells proliferation and development. In another study, David et al. (49) found that the silencing of TAC1 genes by aberrant promoter hypermethylation is now recognized as a critical component in GC initiation and progression. Therefore, these results suggested that the TAC1 gene is considered as a promising biomarker for the early detection of malignancies, independent of clinical-stage, or histological differentiation.
Moreover, Feng et al. (51) indicated that the administration of SKF-96365, a calcium release-activated channel blocker, can suppress the TKs-inducing effects including proliferation, adhesion, migration, and invasion in human GC cells. Additionally, to further support the regulatory role of the SP/NK1R system in GC cells, Rosso et al. (52) showed that L733-060, as a specific NK1R antagonist, dose-dependently induce apoptotic cell death in GC 23132/87 cells. Taken together, these results suggest that targeting NK1R can be a novel and promising therapeutic strategy for GC therapy.
3.4. TKs and Colorectal Cancer
Colorectal cancer (CRC) is one of the most frequent malignant neoplasms and has a relatively poor prognosis, which arises from the lining of the large intestine (53, 54). According to the results of previous studies, TKs and their receptors appear to be overexpressed in mammalian CRC cells, which can be involved in carcinogenesis processes (55-57). In line with this, a population-based case-control study has shown that the TACR1 polymorphism, may act as a genetic susceptibility factor, which could subsequently lead to increased CRC progression and development (58). In malignancies, hypermethylation of promoter site of tumor suppressor genes is commonly observed, leading to the silenced expressions. Reswitching the tumor suppressor gene expression exerts protective effects on cancer progression, which has probably changed the cancer phenotype to normal phenotype. In line with this, Zain et al. (59) showed that methanolic extract of P. debilis decrease the TAC1 gene hypermethylation and subsequently may have a beneficial effect in the long-term through enhancing the abnormal TAC1 gene activity back to normal. Similarly, in another study, Tham et al. (60) found that TAC1 hypermethylation, which has multiple biological activities in CRC pathophysiology, may probably be considered as a useful biomarker for early detection and prediction of recurrence and the survival of patients with CRC. Consistently, Pagan et al. (61) showed that the administration of SR140333 (1 mg/kg), an NK1R antagonist, dose-dependently exerts pro-apoptotic functions via inducing the suppression of extracellular signal-regulated kinases 1 and 2 (ERK1/2) and cyclooxygenase 2 (COX-2) signaling cascades in a rat model of CRC. To further support the regulatory role of TKs in CRC progression and development, Gao et al. (62) indicated that interaction of truncated-NK1R (tr-NK1R), as a dominant form of NK1R, with its ligand probably play an essential role in the tumor microenvironment. In addition, these findings suggested that diminishing the expression of tr-NK1R may be used as a potential therapeutic strategy to prevent dysplasia of the colorectal adenomas from progressing to colorectal carcinomas (62). Besides, another in vitro study presented evidence that NKP608, as a selective and specific NK1R antagonist, leads to induced inhibiting the cell growth, invasion, migration, as well as enhanced programmed cell death in the human CRC cells, via suppressing the Wnt/β-catenin signaling axis (63). Moreover, it has also recently been shown that neuromedin-B (NMB) via binding to specific receptor sites, can up-regulate the growth factors expression and it has even been suggested that NMB may be played a critical role in mediating the colonocyte proliferation (64). Furthermore, Chen et al. (65) indicated that TKs and their receptors are crucial components for CRC development and progression. Taken together, these data suggest that utilizing NK1R antagonists can be considered as a promising new candidate for innovative therapeutic strategies against CRC.
3.5. TKs and Hepatocellular Carcinoma
Hepatocellular carcinoma is the most lethal and common primary malignant tumor of the liver in patients who already have signs of early or advanced chronic liver diseases (66, 67). Several studies are supporting the significantly higher expression of the TACR1 gene in human hepatoblastoma patients compared to healthy control (68-70). For instance, Berger et al. (68) showed that tr-NK1R is highly overexpressed in all hepatoblastoma cell lines. Furthermore, Garnier et al. (69) reported that tr-NK1R overexpression has no significant correlation with the tumor characteristics. Taken together, these findings strongly suggest that tr-NK1R overexpression may be served as a useful marker to predict the prognosis of patients with hepatocellular carcinoma, independent of tumor biology and clinical stage. Consistently, Ilmer et al. (70) showed that administration of aprepitant (40 µM for 24 hours), dose and time-dependently, suppressed the phosphorylation and activation of the canonical Wnt signaling pathway, as well as liver stemness markers, which consequently enhanced liver fibrosis in hepatoblastoma cell lines.
Liver transplantation (LT), which eliminates the hepatic carcinoma and treats the hepatic insufficiency, is the selective treatment of some patients with HCC. In line with this, Lorente et al. (71) found that a positive correlation between increased circulating levels of serum SP before LT and mortality during the first year of LT. Collectively, these results suggest that serum SP levels before LT can be used to estimate one-year survival prognosis.
Additionally, other recent studies have revealed that SP/NK1R system plays a vital role in the cellular senescence of hepatic stellate cells. Consistently, Wan et al. (72) showed that the administration of an NK1R antagonist (20 mg/kg) in a bile duct ligated mice induce hepatic stellate cell senescence, which is associated with reduced liver function, fibrosis, and hepatocellular carcinoma.
3.6. TKs and Cholangiocarcinoma
Cholangiocarcinoma is a rare neoplastic transformation related to epithelial cells, which originates from the bile duct (73). Recently, several lines of evidence have indicated that TK neuropeptides may contribute to cholangiocyte proliferation and metastasis (74, 75). For instance, Deng et al. (76) demonstrated that endogenous agonist SP through binding to NK1R can promote gallbladder cancer cell proliferation, metastasis, and invasion in vivo through modulation of NK1R/Akt/NF‐κB axis.
The SP-degrading metalloproteinases are an intriguing family of enzymes that have been evolved to digest extracellular SP (77). In line with this, Glaser et al. (78) showed that SP/NK1R overexpression is associated with decreased SP-degrading metalloproteinase. These results suggest that SP/NK1R overexpression may act as a progressive role in a cholangiocarcinoma cell line. Consistently, another study on the bile duct ligated mice demonstrated that SP/NK1R overexpression can lead to cholangiocyte malignancy through enhanced cAMP levels, as well as activation and phosphorylation of protein kinase A (78). Therefore, taking the above evidence together, it is deduced that the administration of NK1R antagonist, including aprepitant and L733-060, can reduce the cholangiocarcinoma cell proliferation and development.
3.7. Tks and Pancreatic Cancer
Pancreatic cancer is a GI neoplasm with an abysmal prognosis, deriving from neuroendocrine cells of the pancreas, with a low 5-year survival rate (5%) (79). Some reports have described a positive association between NK1R overexpression and the degree of dysplasia within pancreatic malignancy (80, 81). In line with this, De Araujo et al. (80) showed that SP-radiolabeled with low activity of lutetium-177 (177 Lu-DOTA-SP) have a higher uptake via tumor cells compared to normal groups, suggesting that, the involvement of NK1R distribution in the surface of pancreatic cancer cells. Similarly, in another in vitro study, it is found that SPA, as a potent broad-spectrum GPCR antagonist, significantly attenuates the DNA synthesis as well as tumor proliferation in pancreatic cancer cells (81). Consistent with these experiments, Munoz et al. (82) revealed that the administration of L-733,060 (< 40 µM), dose-dependently can inhibit DNA synthesis and cancer cell progression in human pancreatic ductal adenocarcinoma (PDAC). Additionally, Maekawa et al. (83) indicated that TAC1 promoter hypermethylation values are similar in all stages of pancreatic cancer compared to adjacent tissues. Consequently, the hypermethylation of the TAC1 gene may be considered as a potential biomarker for the diagnosis of pancreatic cancer (83). In a well-established animal model of pancreatic cancer, Sinha et al. (84) demonstrated that robust activation of SP/NK1R/Stat3 signaling cascade enhances pancreatic intraepithelial neoplasia (PanIN) development. As a consequence, this study extended sensory denervation, which could lead to decreased NK1R + PanIN cell progression to PDAC. Furthermore, Amiti et al. demonstrated that menadione (vitamin K3) may exert an anti-inflammatory effect through down-regulating SP/NK1R signaling axis via the NF-κB pathway in a mice model of caerulein-induced Acute pancreatitis (85). In addition, in situ hybridization and immunohistochemistry analysis revealed that NK1R overexpression can also enhance vascular permeability and thereby induce malignant transformation of human pancreatic cancer cells (86).
The up-regulation of matrix metalloproteinases (MMP-2 and MMP-9) have been shown to act as an essential role in the migration and invasion of pancreatic cancer cells (87). In line with this, Li et al. (88) showed that SP, dose, and time-dependently (100 nmol/L for 8 days), increase the MMP2 expression and consequently promotes neurite outgrowth as well as invasion of pancreatic cancer cell cluster to dorsal root ganglions.
3.8. Anti-Carcinogenic Properties of TK Receptor (TKR) Antagonists Against Human Tumors
The primary emphasis of this study was to focus on determining the selective molecules for TKR to provide a comprehensive overview and strategic design for interactions in some biological and pathobiological processes. It should be noted that TKR can mediate several neuropathological processes in the central nervous system, including anxiety, depression, stress, migraine, and emotional behavior. Whereas in the peripheral nervous system, up-regulation of TKR protein expression plays a crucial role in the pathology of autoimmune and inflammatory diseases such as rheumatoid arthritis, multiple sclerosis, psoriasis, and inflammatory bowel disease (3, 89). Recently, several lines of evidence have emerged to suggest that TKR antagonists exert an anticancer action against tumorigenesis (25, 63, 70, 76). For instance, it has also been shown that specific NK1R antagonists are capable of inhibiting the SP-enhanced cancer cell development, cell invasion, and metastasis through the repression of the β-arrestin-containing complex as well as inactivation of the MAPK signaling pathway in many types of tumors (90, 91). Similarly, another in vitro studies have indicated that selective TKR antagonists may play a clinicalrole in modulating antiangiogenic processes via inhibiting hypoxia-inducible factor (HIF-1α) and VEGF mRNA expression in various tumors (92). Besides, acting as a crucial factor during anti-carcinogenesis, TKR antagonists could down-regulate the AKT mRNA and protein levels, which thereby induced caspase 3-dependent apoptosis in human ESCC (39).
Taken together, these results strongly suggest that highly potent TKR antagonists could contribute to understanding or the design of new agents to regulate pathological processes and may offer a therapeutic potential to attenuate cancer proliferation and metastasis.
4. Conclusions
Recently, the regulatory effects of Tks and their receptors on cancer pathology have attracted tremendous attention and have also provided new research areas for therapeutic purposes. TKs mediate various processes, including migration, angiogenesis, apoptosis, and metastasis in gastrointestinal (GI) cancer cells. Several lines of evidence support the role of TKR antagonists as a potential therapeutic approach in cancer therapy. Although the carcinogenic role of the SP/NK1R system has been intensively investigated, the molecular mechanisms of this signaling pathway are poorly understood. Thus, further studies are required to clarify the exact mechanism of the regulatory role of the TK/TKR system on patients with GI malignancies. In this review, we summarized current knowledge about the effects of TKR in the pathogenesis of GI malignancies for a better understanding and hence better management of these cancers. Therefore, understanding the underlying mechanism of the TK/TKR system can help to have a more excellent clinical vision for the treatment of GI cancers.