1. Context
Breast cancer is one of the most common cancers among women. In other words, breast cancer ranked the first among all cancers after age 50 years (1, 2). Generally, breast cancer is the third cause of death in European countries after lung and colorectal cancer and it annually causes more than half a million deaths in this continent (1, 3).
Over 508,000 women died in 2011 due to breast cancer worldwide. Although breast cancer is thought a disease of the developed world, almost 50% of patients with breast cancer and 58% of deaths occur in less developed countries. Incidence rates vary greatly worldwide from 19.3 per 100,000 women in Eastern Africa to 89.7 per 100,000 women in Western Europe. In most of the developing regions, the incidence rates are below 40 per 100,000. The lowest incidence rates are found in most African countries; but, here, breast cancer incidence rates are also increasing (4-7). The total number of patients with breast cancer in Iran is 40,000 and 7,000 new cases are added to this number annually (8).
Aging is considered as the most probable cause of cancer in female population. Family history and lifestyle are other variables that may affect the incidence of breast cancer in this population. Dietary habits, smoking, sedentary, weight gain, and obesity increase the risk of breast cancer (9, 10).
In recent years, the role of free radicals, which are also known as the reactive oxygen species in preventing a variety of diseases, has attracted researchers’ attention (11).
Previous epidemiological studies have investigated the association between the risk of cancer and the intake of fruits and vegetables rich in vitamins and antioxidants, and those findings are mixed (12, 13).
Many systematic reviews and meta-analyses have studied the relationship between antioxidants and their impacts on the prevention of various cancers, including colorectal cancer (14), skin cancer (15), endometrial cancer (16), esophageal cancer (17), gastrointestinal cancer (18), and other cancers (19-22). Greenlee (20) is the first one who systematically examined the role of antioxidants in breast cancer treatment; it is a qualitative study, which was conducted in New York in 2009.
Although there are many studies on the role of antioxidants in treatment of cancer, it seems that the effectiveness of these supplements is still unclear. Several studies have reported contradictory findings on the protective effects of these supplements on breast cancer. The only study that systematically examined these results (23) is somewhat old and has limitations including lack of using statistical quantitative methods. However, this study aimed at investigating the role of antioxidants in prevention of breast cancer and also the impact of these supplements on clinical outcomes of patients with breast cancer who are under treatment (chemotherapy, hormone therapy, etc.). The search for evidence was conducted without any time limitation until 2016. The data were analyzed, using meta-analysis method. It seems that this was the first meta-analysis conducted on safety and efficacy of antioxidants and vitamins in breast cancer. It is hoped that the results of this study help the evidence-based policy-taking and decision-making, using these supplements.
2. Methods
This systematic review and meta-analysis study is based on the Cochrane methodology for these types of studies (24).
The structured question for this review was as follows in Box 1:
| Components of Structured Question |
|---|
| - Population: women with or without breast cancer. |
| - Intervention: vitamin and antioxidant supplements. |
| - Comparator: placebo. |
| - Outcome: breast cancer incidence, adverse effects, quality of life, daily hot-flash score. |
| - Type of studies: randomized controlled trial. |
Components of Structured Question
2.1. Search Strategy and Study Selection
We searched the most important and appropriate electronic medical databases, including MEDLINE, PubMed, Cochrane library, Science Direct, Trip, Google Scholar, Institute of Scientific Information (ISI), SCOPUS, and EMBASE, and the relevant websites were searched without time limit up to November 2016. The MeSH system was used, as well as ‘and’ and ‘or’ between words of the same meaning and concept i.e. breast, cancer, antioxidant, supplement, and vitamin. The extracted articles were organized in Endnote software. After deleting the duplicate articles, 2 reviewers assessed the titles and abstracts of search results independently and selected potentially-relevant studies according to our main question (Box 1). The articles that were deemed to be irrelevant to the research objectives were excluded. Then, the full texts of the selected articles were gathered.
After collecting the articles full text, we reviewed their references.
Those articles that did not possess the inclusion criteria were excluded.
Two separate and independent scholars investigated the articles inclusion criteria, and the differences between them were resolved by discussion or by the third scholar to reach a consensus, if required.
2.2. Selection Criteria of Articles
The main inclusion criteria were the following: 1, Studies that are designed as clinical trials; 2, involve women with or without breast cancer; 3, compare antioxidant supplement and placebo; 4, report breast cancer incidence, adverse effects, and/or quality of life outcomes of participant; and 5, are written in English language. The main exclusion criteria were the following: 1, basic or animal study; 2, review; 3, without relevant data; 4, trials with an unbalanced additional modality between groups; and 5, duplicate articles that have up-to-date versions available. Studies that were not published as full reports were not included.
2.3. Methodological Quality Appraisal
Two separate and independent scholars critically evaluated the quality of articles, and the differences between them were resolved by discussion or by the third scholar to reach a consensus, if required. Cochrane checklist for assessing the risk of bias was used for articles critical assessment. This tool evaluates the articles quality in 7 aspects as low, high, uncertain, and risk of bias (21). Seven mentioned aspects are as follow: 1, random sequence generation; 2, allocation concealment; 3, masking of patients and personnel; 4, masking of outcome evaluation; 5, incomplete outcome data; 6, selective reporting bias; and 7, other risk of bias.
In general, if an article gains more than 70 scores, it will have a high quality. If the score is lower than 50, it will have a low quality. Finally, the scores between 50 and 70 have average quality.
2.4. Information Extraction
A special form was designed in Excel 2013 in order to extract the required data from papers at this stage. General information (Including first authors name, year of publication, journal publisher, and national or state of each article), information related to the paper content and structure (Such as study type, quality level, participants characteristics in each group and interventions description), information related to the outcome (Type of outcome, the dual-mode or continuous outcomes, and following outcomes), statistical information (Such as the sample size in each group, mean and standard deviation for continuous outcomes, the incidence rate in dual mode outcome, the confidence interval, and P value), and any other helpful information were recorded in this form.
We tried to contact the corresponding author when there were problems in some data extraction, especially statistical data.
2.5. Analysis Methods
In this study, the relative risk based on the Mantel-Haenszel method and Fix model were used to estimate the effect sizes of binary consequences. In this method, the relative risk was separately estimated in each study, using the number of events and sample size in each group. After weighing, estimated relative risks in each study were pooled by the Mantel-Haenszel statistical method, and 95% confidence interval for the total relative risk was estimated. The standardized mean difference (SMD), which is based on the inverse-variance method, was used for continuous outcomes.
Chi-square test and I2 statistic were used to investigate the data heterogeneity. If this statistic is greater than or equal to 45%, there will be heterogeneity (25, 26).
For all hypothesis tests, P < 0.05 was considered significantly different. Analyses were conducted, using RevMan version 5.1.3 (24) and comprehensive meta-analysis (CMA) Software.
3. Results
3.1. Study Selection and Characteristics of Included Studies
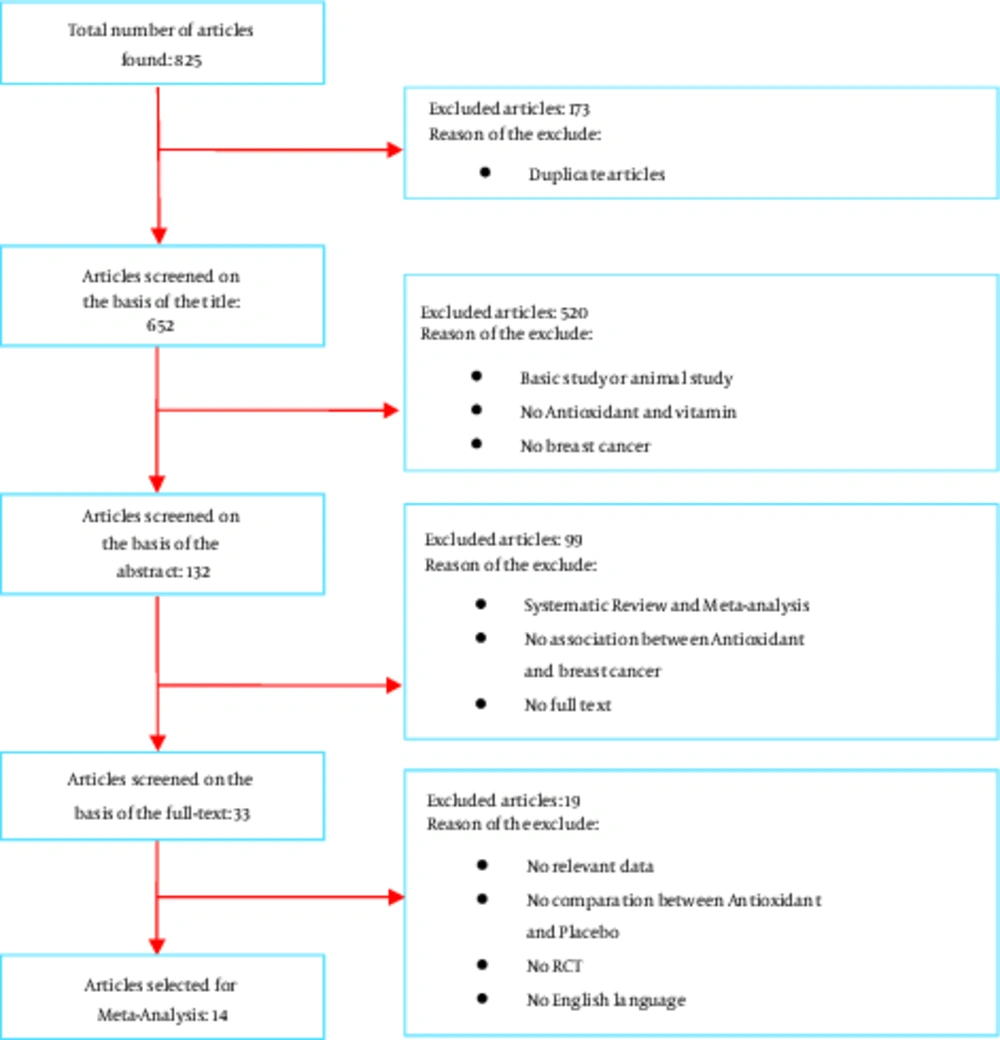
The initial search yielded 825 articles, of which 173 duplicated ones were deleted. Out of the 652 remaining papers, 619 ones were excluded based on the title and abstract. Studying the full report of the 33 remaining papers led to the inclusion of 14 RCT studies. Figure 1 demonstrates the search results and article selection.
Out of 14 analyzed RCTs, 6 articles (24-29) investigated the incidence of breast cancer in women without breast cancer diagnosis. In these 6 articles, the total number of women in Antioxidant group and Placebo group were 45,895 and 4,5981, respectively. The mean age of women was between 45 to 66 years and mean follow-uptime varied between 4.1 and 12.6 years.
Other 8 RCT (30-37) investigated the clinical outcomes among women with breast cancer, who were receiving treatment. In 8 papers, the total number of women in Antioxidant group and Placebo group were 664 and 657, respectively. The age of women was between 18 and 81 years and follow-up time varied between 4 weeks and 36 months. All the including 15 studies were published between 1998 and 2016. The characteristics of the final articles are listed in Table 1.
| Study | Year | Study Type | Age, Y | No. of Patients | Antioxidant Type | Mean Follow-up | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Antioxidant | Placebo | Antioxidant | Placebo | Total | ||||||
| Lee | 2005 | RCT | Mean 54.6 (7.0) | Mean 54.6 (7.0) | 19 937 | 19,939 | 39876 | Vitamin E | 10.1 years | (24) |
| Lee | 1999 | RCT | 45 and older | 45 and older | 19,939 | 19,937 | 39876 | β-Carotene | 4.1 years | (25) |
| HOPE Trial | 2005 | RCT | Mean 66 (7) | Mean 66 (7) | 1,263 | 1,282 | 2545 | Vitamin E | 7 years | (26) |
| Hercberg | 2004 | RCT | Mean 46.6 (6.6) | Mean 46.6 (6.6) | 3,844 | 3,869 | 7713 | Mixed | 7.5 years | (27) |
| Diallo | 2016 | RCT | Mean 47.2 ( 6.6) | Mean 47.2 (6.6) | 751 | 797 | 1548 | Iron intake | 12.6 years | (28) |
| Duffield | 2002 | RCT | Mean 63.4 (10.2) | Mean 63.0 (9.9) | 161 | 157 | 318 | Selenium | 6.4 years | (29) |
| Bezerra | 2007 | RCT | Mean 57.47 (13.46) | Mean 57.56 (15.54) | 19 | 16 | 35 | Centrum Silver | NR | (30) |
| Cheri | 2002 | RCT | Mean 55.5 (6.3) | Mean 54.9 (6.5) | 59 | 64 | 123 | Oral Soy | 4 weeks | (31) |
| Debra | 1998 | RCT | 18 and older | 18 and older | 54 | 50 | 104 | Vitamin E | 9 weeks | (32) |
| Douglas | 2006 | RCT | 27 - 74 | 24 - 73 | 163 | 163 | 326 | Saforis | NR | (33) |
| Glenn | 2013 | RCT | 31 - 85 | 28-72 | 122 | 114 | 236 | CoQ10 | 24 weeks | (34) |
| MacGregor | 2005 | RCT | 37 - 69 | 33 - 70 | 36 | 36 | 72 | Oral Soy | 12 weeks | (35) |
| Lissoni | 1999 | RCT | 42 - 80 | 39 - 81 | 124 | 126 | 250 | Melatonin | 12 - 36 months | (36) |
| Susan | 2000 | RCT | 18 and older | 18 and older | 87 | 88 | 175 | Oral Soy | 9 weeks | (37) |
Characteristics of the Final Articles
3.2. Study Quality
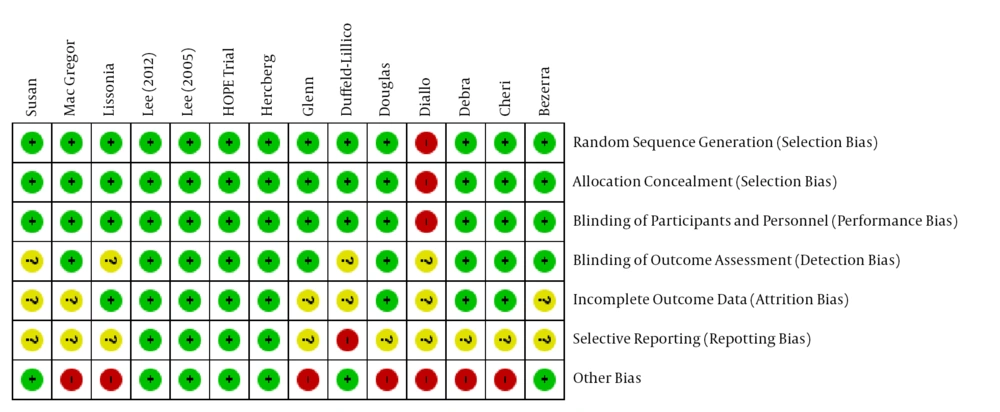
The quality assessment of articles showed that out of 14 articles, 10 had an acceptable quality, 3 had moderate quality, and 1 had low quality (Figure 2).
3.2.1. Breast Cancer Incidence
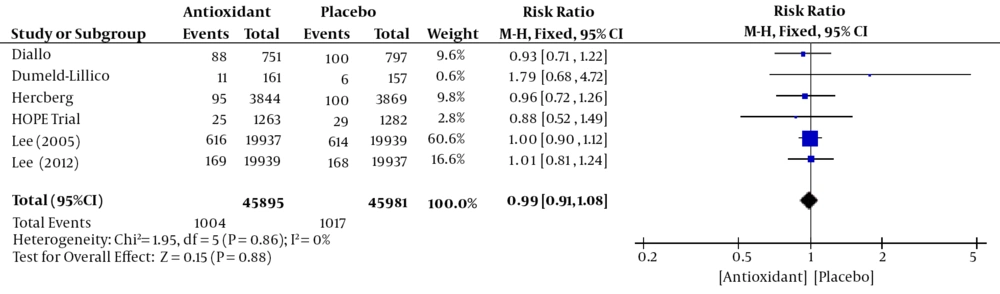
Six studies (24-29) including 9 1876 women (45,895 Antioxidant and 4,5981 Placebo) assessed the breast cancer incidence. The overall Mantel-Hanzel Pooled RR calculated for breast cancer incidence was 0.99 (95% CI; 0.91, 1.08). This difference was not statistically significant (P = 0.88, Figure 3). Test for heterogeneity was not statistically significant (I2 = 0%, P = 0.86).
3.2.2. Quality of Life
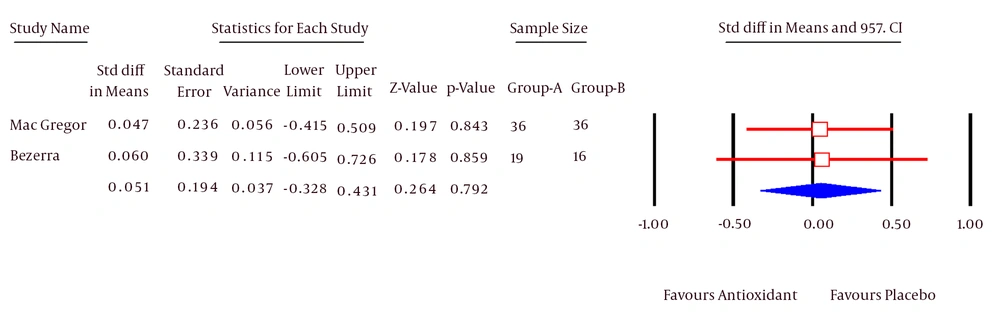
Three RCTs (30, 34, 35) investigated the quality of life in patients with breast cancer. MacGregor and Bezerra used EORTC for measuring the quality of life and Glenn used Fact-B scale for this purpose. Due to different measurement tools in 3 studies, it was not possible to integrate them. Therefore, the studies conducted by MacGregor and Bezerra were integrated and the study of Glenn was separately reported. The mean and standard deviation for each group were not reported in MacGregor’s study. The meta-analysis was not possible with Rev-Man software; therefore, these studies were meta-analyzed, using CMA software.
The Pooled SMD calculated for quality of life was 0.05 (95% CI; -0.32, 0.43). This difference was not statistically significant (P = 0.79, Figure 4). Test for heterogeneity was not statistically significant (I2 = 0%).
In Glenn’s trial, the Fact-B study was used to measure quality of life. The findings showed that the mean of quality of life at the 24th week in antioxidant group and placebo group was 111.9 (SD = 21.2) and 110.4 (SD = 22.1), respectively. The mean difference in these groups is 1.5; this difference is not statistically significant (CI: -4.03, 7.03; P = 0.6).
3.2.3. Daily Hot-Flash Score
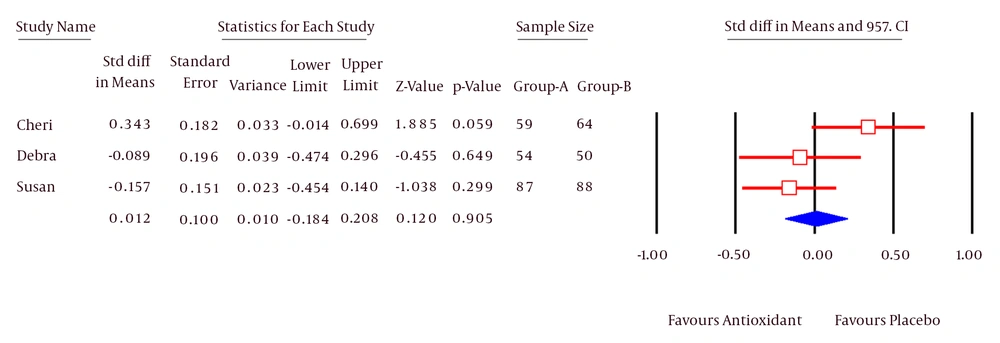
Three studies (31, 32, 37) including 402 patients (200 Antioxidant and 202 Placebo) assessed the daily hot-flash score. The Pooled SMD calculated for Daily Hot-Flash Score was 0.01 (95% CI; -0.18, 0.2). This difference was not statistically significant (P = 0.87, Figure 5). Test for heterogeneity was not statistically significant (I2 = 0%).
3.3. Toxicity
3.3.1. Nausea/Vomiting
Six studies (31-36) including 1497 patients (745 Antioxidant and 752 Placebo) assessed the toxicity-nausea/vomiting outcome. The overall Mantel-Hanzel Pooled RR calculated for nausea/vomiting was 0.98 (95% CI; 0.8, 1.21). This difference was not statistically significant (P = 0.87, Table 2). Test for heterogeneity was not statistically significant (I2 = 7%, P = 0.37).
| Toxicity Type | No Study | Participants | Statistical Method | Effect Estimate | P Value |
|---|---|---|---|---|---|
| Nausea/vomiting | 6 | 1 497 | Risk ratio (M-H, fixed, 95% CI) | 0.98 (0.80, 1.21) | 0.87 |
| Diarrhea | 3 | 609 | Risk ratio (M-H, fixed, 95% CI) | 0.70 (0.42, 1.16) | 0.17 |
| Constipation | 3 | 427 | Risk ratio (M-H, fixed, 95% CI) | 1.67 (0.57, 4.95) | 0.35 |
| Fatigue | 3 | 1 058 | Risk ratio (M-H, fixed, 95% CI) | 0.69 (0.42, 1.13) | 0.14 |
| Alopecia | 2 | 870 | Risk ratio (M-H, fixed, 95% CI) | 0.87 (0.69, 1.09) | 0.22 |
| Anemia | 2 | 486 | Risk ratio (M-H, fixed, 95% CI) | 0.83 (0.36, 1.91) | 0.67 |
| Headaches | 2 | 269 | Risk ratio (M-H, fixed, 95% CI) | 1.11 (0.63, 1.96) | 0.73 |
| Leucopenia | 3 | 1 106 | Risk ratio (M-H, fixed, 95% CI) | 0.68 (0.38, 1.22) | 0.2 |
| Neutropenia | 2 | 856 | Risk ratio (M-H, fixed, 95% CI) | 2.46 (0.90, 6.76) | 0.08 |
| Asthenia | 2 | 870 | Risk ratio (M-H, fixed, 95% CI) | 0.43 (0.31, 0.59) | < 0.00001 |
| Gastritis | 2 | 373 | Risk ratio (M-H, fixed, 95% CI) | 0.37 (0.21, 0.65) | 0.0005 |
Analysis of the Toxicity
3.3.2. Diarrhea
Three studies (31, 34, 36) including 609 patients (305 Antioxidant and 304 Placebo) assessed the toxicity-diarrhea outcome. The overall Mantel-Hanzel Pooled RR calculated for diarrhea was 0.7 (95% CI; 0.42, 1.16). This difference was not statistically significant (P = 0.17, Table 2). Test for heterogeneity was not statistically significant (I2 = 0%, P = 0.69).
3.3.3. Constipation
Three studies (31, 34, 35) including 427 patients (214 Antioxidant and 213 Placebo) assessed the toxicity-constipation outcome. The overall Mantel-Hanzel Pooled RR calculated for constipation was 1.67 (95% CI; 0.57, 4.95). This difference was not statistically significant (P = 0.35, Table 2). Test for heterogeneity was not statistically significant (I2 = 0%, P = 0.71).
3.3.4. Fatigue
Three studies (32-34) including 1,058 patients (532 Antioxidant and 526 Placebo) assessed the toxicity-fatigue outcome. The overall Mantel-Hanzel Pooled RR calculated for fatigue was 0.69 (95% CI; 0.42, 1.13). This difference was not statistically significant (P = 0.14, Table 2). Test for heterogeneity was not statistically significant (I2 = 0%, P = 0.68).
3.3.5. Alopecia
Two studies (33, 36) including 870 patients (430 Antioxidant and 440 Placebo) assessed the toxicity-alopecia outcome. The overall Mantel-Hanzel Pooled RR calculated for alopecia was 0.87 (95% CI; 0.69, 1.09). This difference was not statistically significant (P = 0.22, Table 2). Test for heterogeneity was not statistically significant (I2 = 0%, P = 0.9).
3.3.6. Anemia
Two studies (34, 36) including 486 patients (246 Antioxidant and 240 Placebo) assessed the toxicity-anemia outcome. The overall Mantel-Hanzel Pooled RR calculated for anemia was 0.83 (95% CI; 0.36, 1.91). This difference was not statistically significant (P = 0.67, Table 2). Test for heterogeneity was not statistically significant (I2 = 0%, P = 0.43).
3.3.7. Headaches
Two studies (32, 35) including 269 patients (134 Antioxidant and 135 Placebo) assessed the toxicity-headaches outcome. The overall Mantel-Hanzel Pooled RR calculated for headaches was 1.11 (95% CI; 0.63, 1.96). This difference was not statistically significant (P = 0.73, Table 2). Test for heterogeneity was not statistically significant (I2 = 33%, P = 0.22).
3.3.8. Leukopenia
Three studies (33, 34, 36) including 1 106 patients (552 Antioxidant and 554 Placebo) assessed the toxicity-leukopenia outcome. The overall Mantel-Hanzel Pooled RR calculated for leukopenia was 0.68 (95% CI; 0.38, 1.22). This difference was not statistically significant (P = 0.2, Table 2). Test for heterogeneity was not statistically significant (I2 = 0%, P = 0.65).
3.3.9. Neutropenia
Two studies (33, 34) including 856 patients (428 Antioxidant and 428 Placebo) assessed the toxicity-neutropenia outcome. The overall Mantel-Hanzel Pooled RR calculated for neutropenia was 2.46 (95% CI; 0.9, 6.76). This difference was not statistically significant (P = 0.08, Table 2). Test for heterogeneity was not statistically significant (I2 = 0%, P = 0.51).
3.3.10. Asthenia
Two studies (33, 36) including 870 patients (430 Antioxidant and 440 Placebo) assessed the toxicity-asthenia outcome. The overall Mantel-Hanzel Pooled RR calculated for asthenia was 0.43 (95% CI; 0.31, 0.59). This difference was statistically significant favors Antioxidant (P < 0.00001, Table 2). Test for heterogeneity was not statistically significant (I2 = 0%, P = 0.53).
3.3.11. Gastritis
Two studies (34, 36) including 373 patients (183 Antioxidant and 190 Placebo) assessed the toxicity-gastritis outcome. The overall Mantel-Hanzel Pooled RR calculated for gastritis was 0.37 (95% CI; 0.21, 0.65). This difference was statistically significant and favored Antioxidant (P = 0.0005, Table 2). Test for heterogeneity was not statistically significant (I2 = 57%, P = 0.13).
3.4. Publication Bias
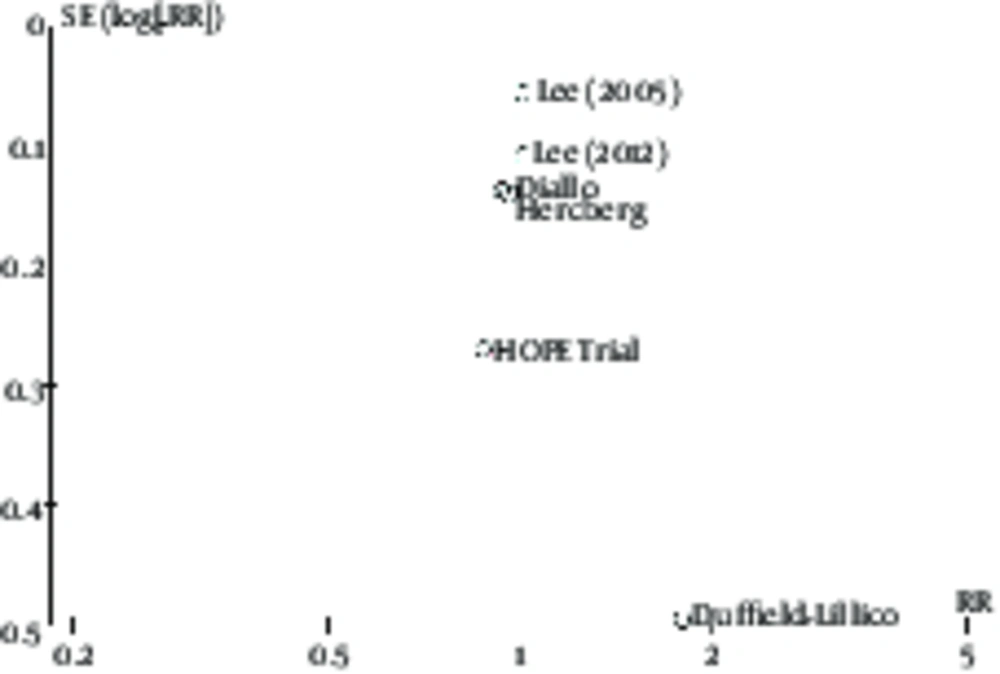
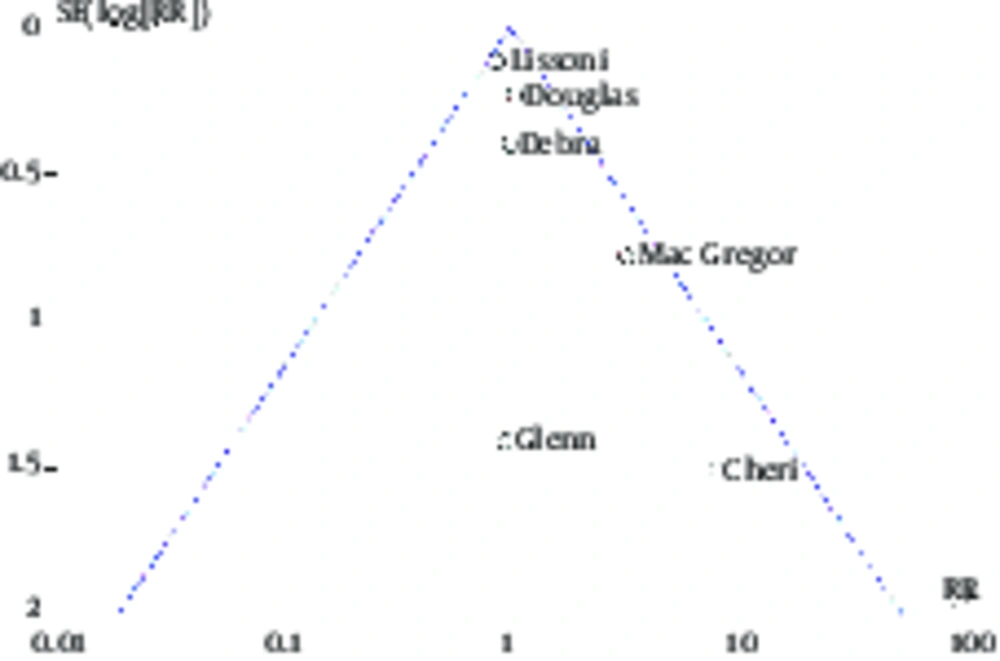
In this study, 6 studies of breast cancer risk and 3 studies of side effects of women with breast cancer undergoing treatment have been investigated. Therefore, the publication bias in these groups of articles was separately evaluated. As shown in Figures 6 and 7, there is no significant difference, indicating the existence of the publication bias in the funnel plot of these two groups of articles and their distribution is almost symmetric.
4. Discussion
Firstly, this study aimed at investigating the preventive role of antioxidants and vitamins in the incidence of breast cancer; in this regard, 6 RCT studies (24-29) were analyzed. Also, this study aimed at investigating the effectiveness of these supplements in women with breast cancer, who were receiving various treatments such as chemotherapy, hormone therapy, and radiotherapy; in this regard, 8 RCT studies (30-37) were analyzed. In all the 14 papers, the antioxidants were investigated compared with placebo. The qualities of these 14 articles were evaluated based on Cochrane’s risk of bias criteria. It was shown that 13 articles had acceptable quality; only 1 article had low quality.
In 6 articles, which examined the preventive role of antioxidants in the incidence of breast cancer, the role of vitamin E, beta-carotene, selenium, iron, and various types of vitamins, and antioxidants was examined in 2, 1, 1, 1, and 1 article, respectively. In this study, regardless of the used antioxidant type, 6 articles were integrated. The overall sample size in these articles was 91 876 participants; they were followed-up in average 4 to 12 years. The meta-analysis of these 6 articles showed that there was no statistically significant difference between people who used antioxidants and those who used placebo (P = 0.88). In addition, none of the 6 articles reported any significant difference between the two groups; all of the 6 articles showed that the antioxidants have no significant role in the prevention of breast cancer in studied population. In this analysis, no heterogeneity was seen (P = 0.86). Considering the relatively high sample size, lack of heterogeneity, and analysis of each of these articles, it seems that the antioxidants and vitamins have no sufficient role in preventing the breast cancer development.
The main objective of other 8 articles was evaluation the clinical outcomes in women with breast cancer, who were receiving treatment (such as chemotherapy, radiotherapy, etc.) and used antioxidants and vitamins. The 10 articles were designed as a randomized trial. The antioxidants, which were used in 3 oral soy articles, included vitamin E in 1 article, melatonin in 1 article, zinc in 1 article, Saforis in 1 article, CoQ10 in 1 article, Centrum in 1 article, and Curcumin in 1 article.
Out of 8 articles, 3 RCTs (30, 34, 35) reviewed the quality of life. MacGregor and Bezerra used EORTC for measuring quality of life and Glenn used Fact-B scale for this purpose. Due to different measurement tools in 3 studies, it was not possible to integrate them. Therefore, the studies conducted by MacGregor and Bezerra were integrated and the study of Glenn was separately reported. The meta-analysis of MacGregor and Bezerra articles with a sample size of 107 patients showed that the outcome was not significant in patients in antioxidant and placebo groups (P = 0.79). On the other hand, Glenn’s trial with a sample size of 236 patients and using Fact-B scale as a measure of quality of life showed that there was no significant difference between 2 groups (P = 0.6). Overall, the results of these 3 articles suggested that the antioxidants do not improve quality of life of patients with breast cancer, who receive chemotherapy, radiotherapy, and other treatments.
Considering the outcome of Daily Hot-Flash, the meta-analysis results of 3 articles (31, 32, 37) with a sample size of 402 patients showed that the outcome was not significant between 2 groups (P = 0.87). Considering the lack of heterogeneity between these articles, it seems that the antioxidants have no impact on improving the symptoms of Daily Hot-Flash outcome in patients with breast cancer.
The reduction of side effects was examined in 6 articles. The meta-analysis results showed that there was no significant difference between antioxidant and placebo groups in nausea-vomiting (P = 0.87), diarrhea (P = 0.17), constipation (P = 0.35), fatigue (P = 0.14), alopecia (P = 0.22), anemia (P = 0.67), headaches (P = 0.73), leukopenia (P = 0.2), and Neutropenia (P = 0.08), except in gastritis and asthenia.
This study had several limitations. Since the findings of this study were based on interventions articles, which examined the role of antioxidants and since the biological activities of supplements are probably different from vegetables and fruits, it is believed that these results cannot be generalized to the role of fruits and vegetables. In examining the preventive role of fruits and vegetables, the observation studies, including treatment-control studies or cohort studies may be used. On the other hand, given that from 6 studies, which were conducted to examine the preventive role of antioxidants, only 2 studies were conducted in healthy female population and other articles were conducted on women at risk or with a history of cancer, the generalization of these results to healthy population should be done with caution.
In 8 RCT studies examining the impact of antioxidants on patients with breast cancer, only the quality of life, daily hot-flash, and toxicity results were common; there was no community in other results in studies. This study aimed at conducting quantitative meta-analysis of outcomes; for this reason, this study did not qualitatively assess other outcomes of these papers. In addition to these 8 articles, other studies were excluded due to lack of criteria and the desired outcome. Another limitation of this study was the short follow-up duration of outcomes in 8 articles, which examined the role of antioxidant in women with breast cancer who were receiving treatment.
Unfortunately, we have just entered studies that were published in English and Persian due to limited time and resources. But, blinding method was used in selection and quality evaluation stages in order to avoid referral bias.
It seems that a systematic review and synthesis of qualitative studies on the role of antioxidants in women with breast cancer is essential to achieve better and more logical conclusions. On the other hand, due to lack of strong and visible evidence to support antioxidants usage, it is recommended that the clinicians prescribe these supplements cautiously, especially in patients with breast cancer who are receiving treatment.
4.1. Conclusions
The current meta-analysis of randomized controlled trials suggests that there is no clinical evidence to support the efficacy of vitamin and antioxidant supplements in reducing the risk or preventive effect of breast cancer. The evidence is currently insufficient to inform clinician and patient guidelines on the use of antioxidant supplements during the breast cancer treatment. The potential effects (either beneficial or detrimental) of antioxidant supplements on human health, particularly in relation to breast cancer, should not be overemphasized. The findings and explanations presented in this study should be explored in future research. Thus, well designed clinical trials and observational studies are needed to determine the short- and long-term effects of such agents.