1. Background
The history of cancer treatment has been entwined with the efforts to find a susceptible point to stroke and knockdown cancer cells with the hope to finally conquer the disease. Long before the identification of the role of signaling pathways in the pathogenesis of human malignancies, cancer was a dreadful beast to fight with (1). The advent of the first small molecule inhibitors which could directly target a specific component of a signaling pathway in leukemic cells has changed the landscape of the conventional therapeutic strategies opening up the opportunities for these molecules to find their way into novel protocols (2). Because of the prevalent aberrancy in malignant transformation and based on their multifunctional property, cyclin-dependent kinases (CDKs) are among those components that are believed to be a promising target for drug design (3). The first generation of CDK inhibitors, which mainly serve as non-specific agents, did not end well in the clinical trials; however, the more the knowledge about the biology of CDK was progressed, the more specific CDK inhibitors with better safety profile were developed in parallel (4).
AT7519, an inhibitor of several CDKs, has shown to be a promising agent in inducing cell death in a wide range of solid tumors such as neuroblastoma (5), colon (6), and cervical (7) cancers. A study conducted by Santo et al. indicated that AT7519 exerted a favorable effect in multiple myeloma cells via the suppression of glycogen synthase kinase-3β (GSK-3β) phosphorylation (8). In another study, Squires et al. (9) reported that the inhibition of CDK in chronic lymphoblastic leukemia (CLL) cells is coupled with the induction of apoptosis, regardless of the Rai stage, through alteration in the expression levels of anti-apoptotic genes. What made AT7519 a more potent inhibitor in human cancer investigations was its safe pharmacokinetic activity which was evaluated under several clinical trials. Even at concentrations below its maximum tolerated dose, AT7519 was capable to induce an anti-cancer effect in several drug-resistant solid tumors (10). This remarkable feature was not restricted only to the application of AT7519 as a single agent since the results of Phase II clinical trial in multiple myeloma (MM) patients showed that the synergism of this inhibitor with bortezomib was well tolerated in patients (11).
2. Objectives
Although the findings of the laboratory bench and bedside treatment suggested AT7519 as a valuable agent in cancer treatment strategies, the procedure of action of this CDK inhibitor in leukemic cells has not been entirely elucidated. Here, we showed that CDK suppression in acute myeloid leukemia (AML) - derived U937 cells could reduce the survival rate of the cells not only by accumulating cells at the G2/M stage of cell cycle but also through the apoptosis initiation. Moreover, we proposed that the effectiveness of this drug in acute leukemia cells could be blunt through either the overexpression of c-Myc oncogene or the activation of the autophagy system.
3. Methods
3.1. Chemicals and Cell Culture Procedure
The inhibitors of CDK (AT7519) and c-Myc (10058-F4) were prepared from Selleckchem (Munich, Germany). For stock preparation, both agents were first dissolved in dimethyl sulfoxide (DMSO) and then were diluted with RPMI 1640 medium to reach the desired concentration. Additionally, vincristine (VCR) and autophagy inhibitor chloroquine (Sigma, Germany) was used in this study for further experiments. To evaluate the effect of AT7519 on acute myeloid leukemia, U937 cells were proliferated in RPMI 1640 medium and then were treated with different concentrations of the agent.
3.2. Evaluation of Cell Cycle Progression Using Flow Cytometry
PI staining was used to evaluate the impact of CDK inhibition on how cells transit from one phase of the cell cycle to another. After incubating U937 cells with desired concentrations of AT7519, cells were harvested by using centrifugation and then were fixed by 70% ethanol. Then, PI and RNase were added to the fixed cells to mark DNA and destroy RNA, respectively. The progression of the cell cycle was examined and interpreted by flow cytometry and Windows FlowJo V10 software.
3.3. Trypan Blue Exclusion Assay
U937 cells at the primary concentrations of 150 × 103 cells were cultured and the medium containing several doses of AT7519 including 0, 100, 175, 250, and 500 nM, either as an agent alone or in co-treatment with 10058-F4 or VCR was added to U937 cells. At each time interval, cells drug-treated cells were harvested and stained with 0.4% trypan blue for about 2 min at 20°C - 22°C. Then, the whole number of viable and dead cells was calculated under a light microscope to estimate the percentage of viable cells.
3.4. Tracing of Cell Metabolism by MTT Test
Leukemic cells were distributed in a plate with 96 wells, in which the medium containing different concentrations of AT7519, VCR, or 10058-F4 was added previously. After the indicated time interval, 100 µL of MTT solution made by dissolving 5 mg 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide powder in 1 milliliter of PBS, was added to the wells one by one and the plate was incubated for a further 3 hours in a humidified incubator. The plate was then centrifuged and after discarding the medium, 100 µL of DMSO was added to each well to dissolved formazan. The final optical densitometry (OD) was estimated by ELISA reader and the percentage of cell metabolism was calculated by dividing the OD of the test by the OD of the control group, multiplied by 100.
3.5. Evaluation of Gene Expression Using Real-time PCR Analysis
RNA isolation kit (Roche, Mannheim, Germany) and cDNA Synthesis Kit (Takara Bio, Shiga, Japan) were used for extracting RNA from drug-treated U937 cells and to synthesis cDNA, respectively. To evaluate the alteration in gene expression level, the quantitative real-time PCR (qRT-PCR) was carried out with a light cycler instrument using SYBR Premix Ex Taq technology (Takara Bio, Inc). 2-ΔΔCt relative expression formula was used to compute the fold change values. The primers utilized in the present research are summed up in Table 1.
| Gene | Forward Primer (5’ - 3’) | Reverse Primer (5’ - 3’) | Size, bp |
|---|---|---|---|
| ABL | CTGAAGCTGGTGGGCTGCAAATC | CACTCAGACCCTGAGGCTCAAAG | 115 |
| Survivin | CCAGATGACGACCCCATAGAG | TTGTTGGTTTCCTTTGCAATTTT | 152 |
| p27 | AACGTGCGAGTGTCTAACGG | CCCTCTAGGGGTTTGTGATTCT | 139 |
| c-Myc | CCACAGCAAACCTCCTCACAG | GCAGGATAGTCCTTCCGAGTG | 105 |
| PUMA | GACCTCAACGCACAGTACGAG | AGGAGTCCCATGATGAGATTGT | 98 |
| FOXO3a | ACGGCTGACTGATATGGCAG | CGTGATGTTATCCAGCAGGTC | 85 |
| Bax | CGAGAGGTCTTTTTCCGAGTG | GTGGGCGTCCCAAAGTAGG | 242 |
| Pin1 | AGGCAGACTCGAGGGCCGAA | TGCTCAGAGGGCCCATGGCT | 100 |
| CIP2A | ACCCCAACATAAGTGCTTCAC | TCTCTGGTTTCAATGTCTACTGC | 79 |
| p21 | CCTGTCACTGTCTTGTACCCT | GCGTTTGGAGTGGTAGAAATCT | 130 |
3.6. Apoptotic Characteristics Measured by Flow Cytometry
Annexin V and propidium iodide labeling was used to study whether AT7519 could induce apoptotic cell death in U937 cells. After 24 hours, the harvested drug-treated U937 cells were added to incubation buffer and annexin-V-Fluos stain for about 20 min in the completely dark room. Then, flow cytometry was done to measure how much light is emitted.
3.7. Combination and Dose Reduction Index Calculation
CalcuSyn software was used to address this question that is there any synergistic effect between AT7519 and VCR? If the calculated CI value is less than 1, it is representative of the synergistic effect and if it is more than 1, it represents the antagonism effect.
3.8. Acridine Orange Staining Assay
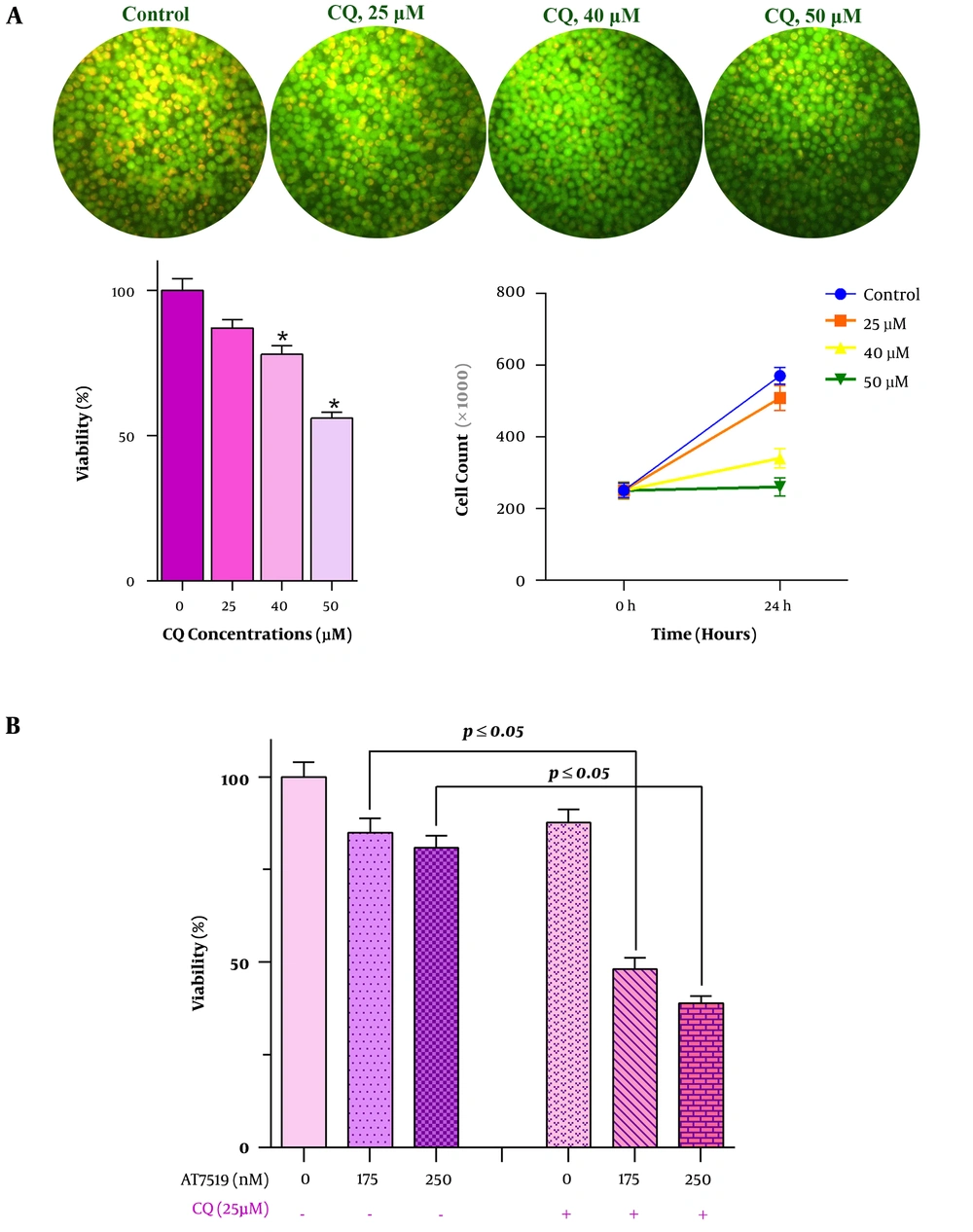
The autophagy impact on the survival rate of U937 cell line was evaluated by treating the cells with different concentrations of CQ. Then, to ascertain that the inhibition of autophagy would enhance the cytotoxic effects of AT7519, U937 cells were treated with CDK inhibitor in combination with CQ for 24 hours. Afterward, the cells were centrifuged and washed twice with PBS. One µg/mL acridine orange dye produced by Merck company, was then added to the cell pellet and the suspension was kept in dark for 15 min. By using a fluorescence microscope (Labomed, Los Angeles), the activation of autophagy within the cells was examined.
3.9. Statistical Analysis
The significance of the results was scrutinized with GraphPad Prism software (one-way variance analysis and two-tailed student’s test). The comparison between the results of the treated and untreated groups was done using Dunnett’s test. Each test was done three times. A P value less than 0.05 was deemed statistically significant and shown by a star in the graphs (*).
4. Results
4.1. U937 Leukemic Cells Viability Was Diminished During A Specific Time Using Various Doses of AT7519
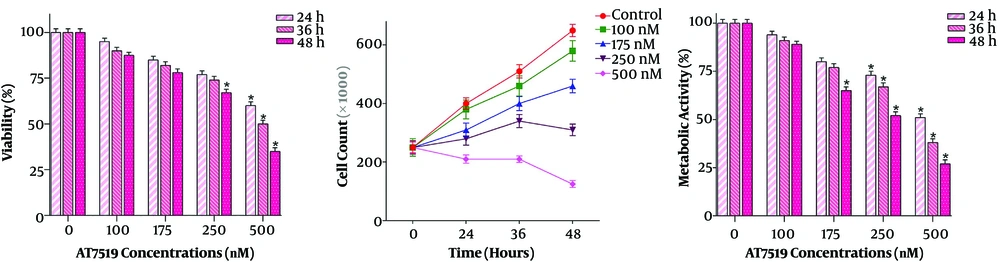
Does the inhibition of cyclin-dependent kinase activity diminish the U937 cells survival? To address this question, several doses (100 - 500 nM) of pan-CDK inhibitor AT7519 were added to U937 cells and then both trypan blue and MTT assays were done after different time intervals (24 h, 36 h, and 48 h). It has been shown in Figure 1 that not only this drug inhibited cell survival in a concentration-dependent manner, but also reduced the proliferation of U937 cells. The cytotoxic effect of the inhibitor was further confirmed by results achieved from the MTT assay. As presented in Figure 1, time-dependent experiments outlined that AT7519 could inhibit metabolic activity of the cells, with maximal suppression observed at 500 nM after 48 hours’ treatment. Given the significant reduction of the survival of U937 cells in the presence of AT7519 at the concentrations of 500 nM, we used this concentration of the agent for conducting the molecular experiments. Overall, the results suggested that the inhibition of CDK induced a significant cytotoxic effect in AML-derived U937 cells; shedding light on the critical role of these regulatory proteins in leukemic cell survival.
4.2. Anti-proliferative Effect of AT7519 Resulted in the Accumulation of the Cells in the G2/M Stage of the Cell Cycle
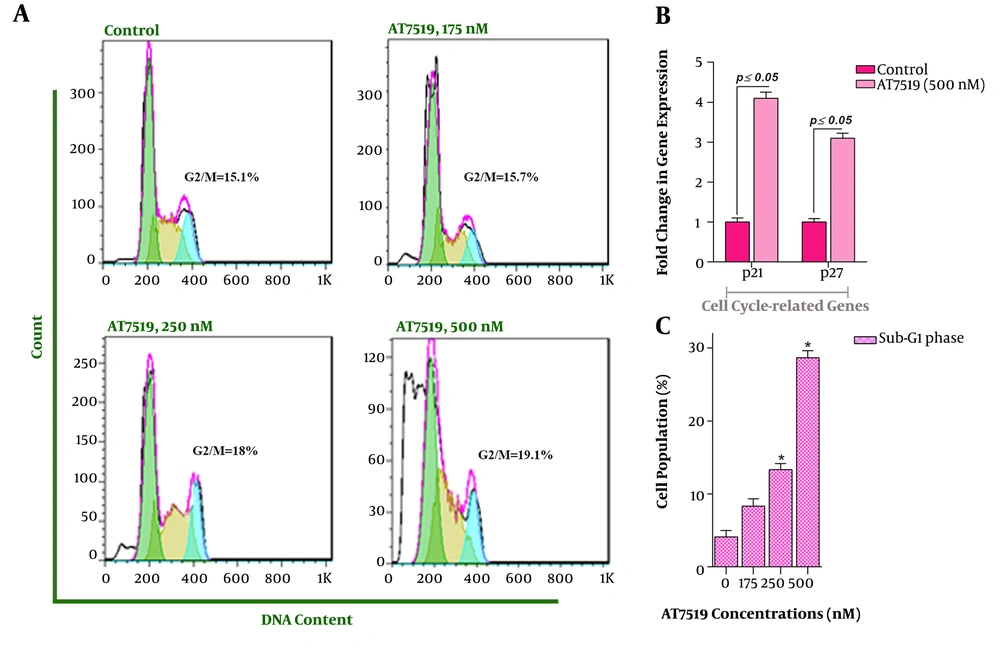
In the light of the anti-proliferative effects of AT7519 and given the crucial role of cyclin-dependent kinases in fine-tune regulation of cell cycle (12), the effect of the inhibitor on the progression of the cell cycle in U937 cells was examined. We found that not only AT7519 diminished the number of the cells in the S phase but also accumulated the cells in G2/M (Figure 2A). Since p21 and p27 cyclin-dependent kinase inhibitors are components of DNA damage-induced cell cycle arrest (13), it was of great interest to examine the mRNA expressions of the aforementioned genes upon treatment with the inhibitor. Intriguingly, the results declared that 48 hours of incubation with AT7519 resulted in a significant induction of p21 and p27 mRNA levels with a nearly 4.1- and 3.1-fold increase at the concentration of 500 nM (Figure 2B). Noteworthy, sub-G1 cell population was augmented through treating the cells with various concentrations, highlighting the ability of this agent to induce apoptotic cell death in U937 cells (Figure 2C).
The impact of AT7519 on the dissemination of the cells in each phases of the cell cycle. A, DNA content analysis revealed that AT7519 could accumulate the cells in G2/M phase; B, U937 cells treatment with AT7519 resulted in the elevation in the expression level of p21 and p27; C, Upon exposure of the cells to the various doses of the CDK inhibitor, the amount of cells in the Sub-G1 phase was augmented considerably. (*, statistically significant).
4.3. AT7519-induced Apoptosis in U937 Cells Was Coupled with the Alteration in Apoptosis-related Genes
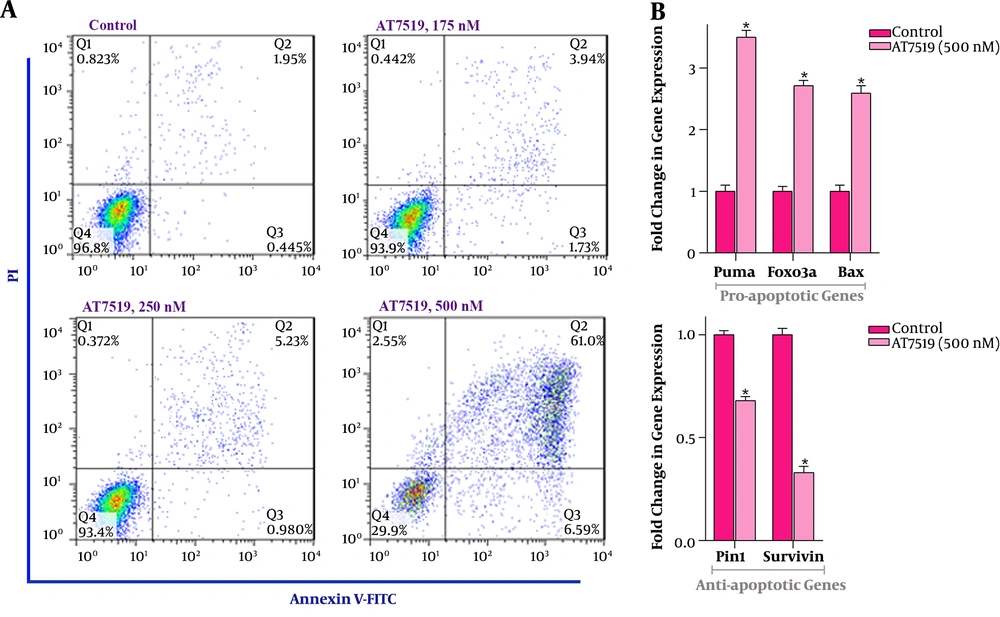
The results showed a substantial augmentation in the amount of both Annexin-V cells and Annexin-V+/PI+ ones. As represented in Figure 3A, the treatment of U937 cells with the inhibitor led to concentration-dependent apoptosis, with maximal apoptotic cells observed at the concentration of 500 nM. For shedding light on the mechanism through which AT7519 could induce apoptotic cell death in leukemic cells, the expressions of apoptosis-related genes were investigated using qRT-PCR analysis. Accordingly, we found that AT7519 up-regulated the expression of pro-apoptotic genes, such as Bax, PUMA, and FoxO3a with a nearly 2.5-, 3.5- and 2.7-fold increase, and inhibited the transcriptional activity of Pin1 and Survivin with approximately 0.6- and 0.3-fold reduction, respectively (Figure 3B). Taken together, the results suggest that AT7519 induced its cytotoxic effect in U937 through the induction of apoptotic cell death, which was coupled with the alteration of the balance ratio between death-repressor and death-promoter genes.
4.4. Suppression of Autophagy in U937 Cells Enhanced the Anti-leukemic Effect of AT7519 in U937 Cells
The results of the previous study declared that the activation of the autophagy system in some cancers may act as oppose to the anti-cancer agents by attenuating their cytotoxic property (14). On the contrary, other studies suggest that autophagy could be exploited by some chemotherapeutic drugs to induce cell death in neoplastic cells (15). Given this, it was of particular interest to evaluate whether the anti-leukemic effect of AT7519 in U937 could be influenced by the alteration of the autophagy system. We incubated U937 cells with the increasing concentrations of an autophagy inhibitor CQ, either alone or in combination with AT7519, then trypan blue assay was applied to investigate the effect of autophagy inhibition on the anti-leukemic effect of the drug. As displayed in Figure 4A, the autophagy blockade in U937 cells, as evident by the reduction in the absorbance of acridine, could reduce the survival of cells; indicating that the suppression of this system may act on the behalf of cell death initiation in this cell line. Notably, the unfavorable impact of autophagy on AT7519 cytotoxicity became even more evident when the results suggested that autophagy inhibition could enhance the anti-leukemic effects of AT7519 (Figure 4B), outlined the impact of autophagy in favor of survival on U937 cells.
Higher anti-leukemic effect of AT7519 plus the blocker of autophagy. A, Suppression of autophagy in U937 cells by chloroquine (CQ), as discovered by a crystal clear alteration in the fluorescence intensity ratio (FIR), resulted in reduction of both viability and metabolic activity of leukemic cells; B, when this system was inhibited in U937 cells, AT7519 induced more prominent anti-leukemic effect on the cells. (*, statistically significant).
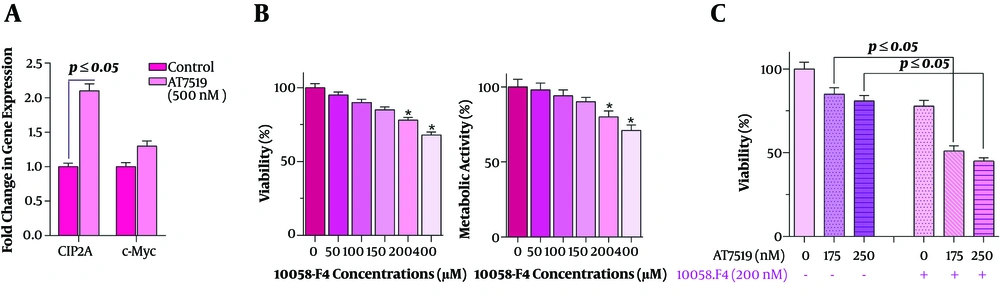
4.5. AT7519 Suppressed the Expression of C-Myc and Its Associated Genes in U937 Cells
As one of the main mediators which play a critical role in the regulation of cell proliferation, CIP2A has attracted tremendous attention in recent years (16). Given the results of qRT-PCR indicating that AT7519 could increase the expression of CIP2A in U937 cells (Figure 5A), we became curious to assess the transcriptional activity of a well-known regulator of the cell cycle, c-Myc, which has a tight association with CIP2A (17). As shown in Figure 5A, we found that this agent could not diminish the expression of c-Myc in U937 cells, suggesting that probably c-Myc could play as a compensatory molecule in the AT7519-induced cytotoxic effect. This hypothesis was further confirmed by the results of combination treatment where we found that the blockage of c-Myc activity using an excel c-Myc blacker 10058-F4 could sway the anti-cancerous impact of AT7519 (Figure 5B). As presented in Figure 5C, both the survival and proliferative rate of U937 cells are diminished more profoundly when AT7519 was used in combination with 10058-F4.
The impact of c-Myc inhibitor on AT7519 anti-leukemic effects. A, Although AT7519 could effectively increase the expression level of CIP2A, the transcriptional activity of c-Myc was not altered meaningfully; B, the c-Myc Inhibition in U937 cells using 10058-F4 was coupled with the reduction in the survival rate of the leukemic cells; C, the anti-leukemic effect of AT7519 was potentiated in the presence of c-Myc inhibitor, indicating that the suppression of c-Myc could ameliorate the efficacy of CDK inhibitor.
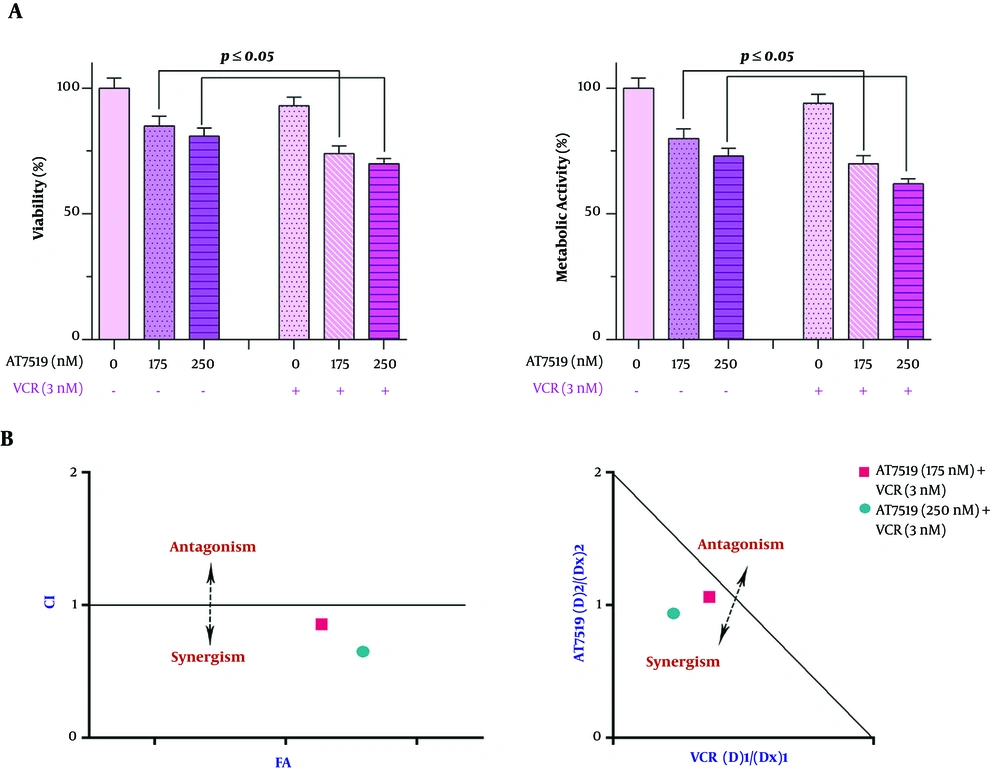
4.6. Combination of AT7519 With VCR Inhibited U937 Cell Proliferation more Profoundly in Comparison with Either Agent Alone
To demonstrate whether CDK suppression could reinforce the anti-cancer activity of vincristine (VCR) in AML cells, the cells were exposed to VCR alone and treated with VCR plus AT7519, as well. As presented in Figure 6, the co-treatment strategy was more satisfactory in impeding U937 cell survival in comparison with every single drug. CI and DRI were measured based on the Chou-Talalay combination index equation to examine if the interactions between AT7519 and VCR were synergistic or caused by additive drug interaction. As presented in Figure 6, the results of CI and isobologram analysis strengthened the existence of synergism between AT7519 and VCR as all the calculated points were plotted under the additive line. The CI and DRI results following 24-hour exposure of the cells to the agents were summarized in Table 2.
Figure 6. Evaluating the synergistic influence between AT7519 and vincristine (VCR). AT7519 could amplify the anti-cancer impact of VCR in U937 cells. Both CI and isobologram stressed the considerable degree of these two drugs interaction.
| AT7519 | VCR | CI | ||
|---|---|---|---|---|
| Dose, µM | DRI | Dose, nM | DRI | |
| 150 | 1.734 | 2 | 4.818 | 0.784 |
| 200 | 1.947 | 2 | 16.541 | 0.574 |
5. Discussion
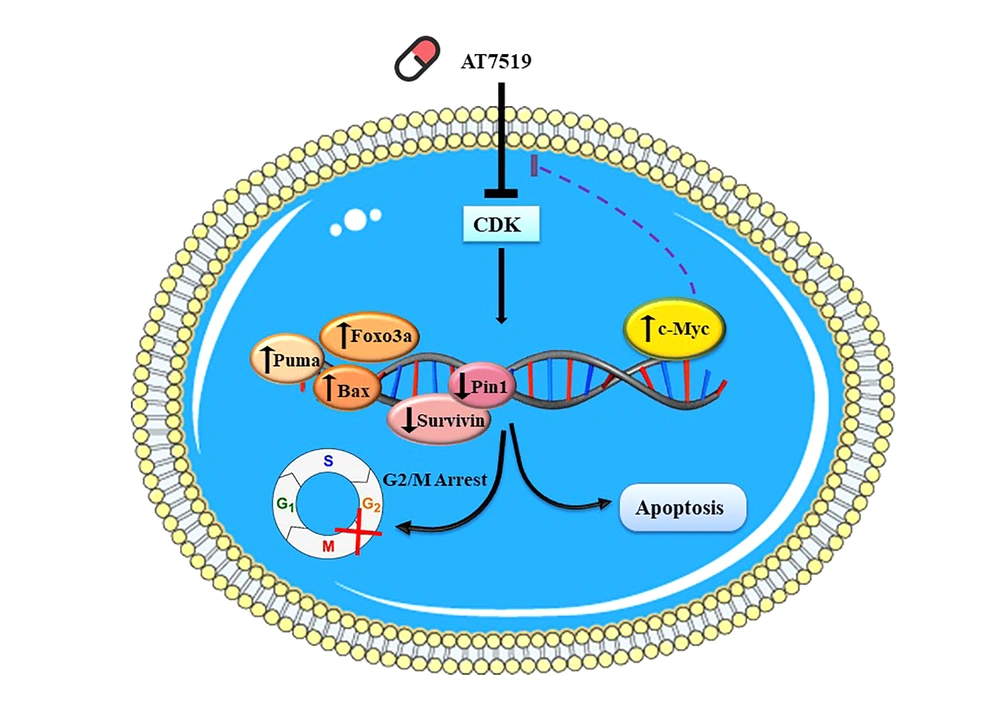
Within the vast array of methods in the category of contemporary cancer treatments, targeting the specific growth stimulatory pathways found to be not only an effective strategy to stroke the malignant nature of tumor cells but also a promising tactic to tackle the tragic event of chemo-resistance. Since the central role of cyclin-dependent kinases (CDK) in the regulation of the cell cycle has been established, mounting efforts have been put off to find an association between these enzymes and the progression of human cancers hoping that this discovery may lead to the advent of prodigious anti-cancer agents (18). Conspicuously, this long way has finally ended with the introduction of several novel small molecule inhibitors with the ability to bind to the different classes of CDKs preventing their ability to construct a complex with their partners, cyclins (4). This study was aimed at not only evaluating the effect of a pan-CDK inhibitor AT7519 on the survival of acute myeloid leukemia-derived U937 cells but also at scheming a molecular mechanism of action for this inhibitor (Figure 7). Noteworthy, the results indicated that the CDK suppression in U937 lessened the proliferative capability of cancerous cells by halting the transmission of the cells from the G2/M phase. This finding was consistent with the results of the previous studies indicating a growth-suppressive property for CDK inhibition both in hematologic malignant cells (9, 19) and solid tumors (5, 7). The results of a recent study also declared that suppression of CDKs using AT7519 inhibits cell cycle progression of AML-derived KG1 cells through the inhibition of cell cycle-related genes (20).
Probable theory behind the mechanisms of action of AT7519 in U937 cells. Upon blockage of multi CDKs in U937 cells, cell transition arrest in G2/M phase through enhancing p21 and p27 transcriptional activity. Besides, the prominent cytotoxic effect of this agent on leukemic cells was together with the apoptotic induction via changing balance between the pro- and anti-apoptotic genes. The efficacy of AT7519 could be partially overshadowed by the over-expression of c-Myc activation in U937 cells, suggesting that co-targeting both c-Myc and CDK would be a better approach for the therapeutic strategy of acute leukemia.
The connection between cell cycle dysregulation and the disturbance of the apoptotic events has lately become an area of focus in recent investigations (21). Accordingly, previous studies have reported numerous non-canonical characteristics for cyclin-dependent kinase inhibitors p21 and p27, which could trigger apoptotic signals leading to induction of programmed cell death through interaction with pro-apoptotic target genes of p53, PUMA, and Bax, (22). In agreement, we found that the anti-survival effect of AT7519 on U937 cell line, as uncovered by the raise in the sub-G1 phase cell population, was together with the apoptotic cell death initiation not only through the increment of the expression of genes in favor of apoptosis but also through diminishing the mRNA levels of both Pin1 and Survivin. Upon suppression of various mediators foremost Pin1, CIP2A is assumed to refrain the cell cycle from progression through the suppression of c-Myc (23). In contrast to the inductive effect of AT7519 on CIP2A, we were disappointed to discover any suppressive impact on the c-Myc expression in U937 cell line. As an asset to the survival of malignant cells, the look toward c-Myc has altered drastically in recent years and what new investigations are claiming about this oncogene is mostly summarized in the chemo-resistance process (24). Likewise, c-Myc acts as a prognostic label in several leukemia patients and up-regulation of this gene is associated with disease relapse (25, 26). Noteworthy, when we blocked the expression of c-Myc using 10058-F4, the anti-cancerous impact of AT7519 was boosted strenuously; indicative of the weakening impact of c-Myc on the effectiveness of CDK inhibitor. Other studies also declared that c-Myc blockage in leukemic cells can be an effective approach to ameliorate the cytotoxic effect of anti-cancer agents while reducing their toxic concentrations (27-31).
As another potent mechanism involved in the acquisition of chemo-resistant phenotype in cancer cells, the autophagy system has long been under intense scrutiny (32). A previous study showed that autophagy could integrate with p53-related proteins and in turn trigger the apoptotic pathway (33). However, other studies deduced that the tumor-suppressive mode of autophagy is overrated in recent investigations, as this mechanism may target the PI3K cascade to amplify the apoptotic pathway (34). Remarkably, the results of our study revealed that hindrance of autophagy using CQ diminished the survival of the leukemic cells on one hand, and on the other hand, increased the cytotoxic effect of AT7519 in AML cells; suggesting an attenuating impact of autophagy against AT7519-induced cell death in U937. Finally, to examine whether the inhibition of CDK could enhance the sensitivity of AML cells to chemotherapeutic drugs used in AML treatment, we examined the effect of AT7519 and vincristine (VCR) combination in U937 cells. We found that the lower doses of VCR in co-treatment with AT7519 produce a synergistic anti-leukemic impact; indicating that the application of small-molecule inhibitors of the cell cycle, like AT7519, in a co-treatment strategy with vinca alkaloids might provide a novel perception about the development of new combination therapies in this leukemia.
5.1. Conclusions
Despite exponential studies to make treatment strategies get better and better, there are still numerous unanswered questions and gaps in the way to reach complete remission for AML patients and this malignancy remains one of the leading causes of person-years of life lost worldwide. Given the importance of CDKs in the regulation of the cell cycle and based on their frequent aberrancies in AML, it is reasonable to assume these molecules as promising targets. All in all, this study proposed that targeting CDKs using AT7519 could probably unwind the complexity of therapeutic obstacles on the way of acute leukemia either in the context of mono- or combined-modal strategy; however, further studies should be performed to determine the beneficial effects of this agent in the clinical setting.