1. Introduction
Secondary involvement of kidney in advanced Non Hodgkin lymphoma is quite common but PRL is a rare disease (1). By definition, the term PRL is applied to patients with disease localized to the kidney without any sign of other organ involvement or in which renal involvement is the presenting manifestation (2). Renal lymphoma occurs throughout life but it is characterized by a peak incidence in the late sixth decade with male predominance. Signs and symptoms of primary renal lymphoma include abdominal mass, weight loss, back and flank pain, hematuria, anorexia and acute renal failure. Renal large B cell lymphoma is more common than other Non-Hodgkin Lymphomas. To our knowledge, primary MCL, pleomorphic variant has not been reported so far. MCL is an aggressive type of lymphoma with poor prognosis and median overall survival is 2 to 5 years. Common extra nodal sites of disease are gastrointestinal tract, bone marrow, liver, spleen and Waldeyer’s ring. MCL has a broad spectrum of different histopathological subtypes, according to World Health Organization (WHO) classification, two aggressive variants of MCL are recognized: blastoid and pleomorphic (3). We present a 67-year-old male with primary renal MCL, pleomorphic variant manifested by right flank pain.
2. Case Presentation
A 67-year-old male with right flank pain referred to our hospital. He did not present any B symptoms. The examination showed a large mass on the right side of abdomen and right varicocele grade II. No peripheral lymphadenopathy or hepatosplenomegaly was detected. The ultrasonography revealed a large right kidney mass with renal distortion measuring 13 × 13 cm accompanied by renal vein thrombosis extending to the IVC. Computed Tomography (CT) and Magnetic Resonance Imaging (MRI) showed right kidney involved by a bizarre shaped soft tissue mass measuring 180 × 133 × 108 mm, xanthogranulomatous pyelonephritis was the first consideration along with probable renal mass. The left kidney appeared normal. No detectable lesion on chest CT scan was seen. Bone scan depicted active lesions in 10th right rib and T11 vertebra which indicated metastasis. Laboratory tests showed white blood cells counting 9 × 109 / L (lymphocytes 9.4 %; granulocytes 76.8 %, MXD 13.8 %); platelet count 167 × 109 / L, hemoglobin 10 g/dl, MCV 85 fl; lactate dehydrogenase 680 U/L (normal range 250 - 480 U/l); and serum creatinine 1.5 mg/dl. The patient was admitted for right nephrectomy. During the surgery, it seemed that the mass had been extended to the right colon; therefore, right hemicolectomy was also done. After preparing pathologic results the patient was admitted in another hospital for continuing treatment and bone marrow biopsy was performed there which was negative for any neoplastic infiltration.
This study and evaluation of patient information has been done by his informed consent with no additional cost imposed.
2.1. Pathologic Findings
2.1.1. Macroscopy
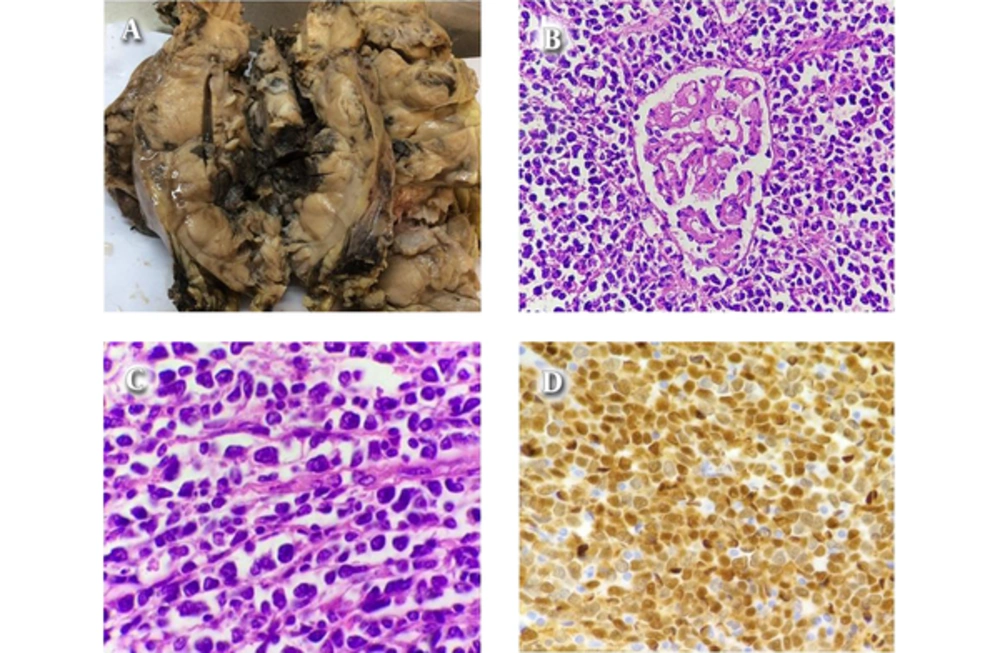
We received kidney with the mass and right hemicolectomy specimens in two formalin containers. The former was a deformed kidney almost totally involved by a poorly defined tan tumor extended to perinephric fat measuring 25 × 18 × 12 cm with areas of tumor rupture. There was a rim of remained renal tissue in one side of the tumor. The cut surfaces revealed solid nodular pattern with areas of hemorrhage (Figure 1A). Adrenal gland was not resected. Also a lymph node measuring 0.7 cm in greatest dimension on renal hilum was also identified.
2.1.2. Microscopy
H & E prepared sections of renal mass showed diffuse infiltration of renal tissue by atypical lymphoid cells mainly composed of large sized cells with pleomorphic nuclei, distinct nucleoli and pale eosinophilic cytoplasm admixed with some medium sized cells with convoluted nuclei, indistinct nucleoli and scant cytoplasm. The mitotic figures were easily found (Figures 1B and 1C). No tumor necrosis and lymph- vascular invasion were seen. Immunohistochemical studies were performed on 3micron sections of formalin fixed paraffin embedded tissue with appropriate positive and negative controls. All the antibodies were from Dako company. The atypical lymphoid cells expressed CD20 and Cyclin D1 (Figure 1D) but were negative for Pan cytokeratin, Myogenin, Myo D1, CD99, CD5, CD3, Chromogranin and NSE. Immunohistochemistry for Ki - 67 (proliferative index) was about 90 %. The hilar lymph node was reactive.
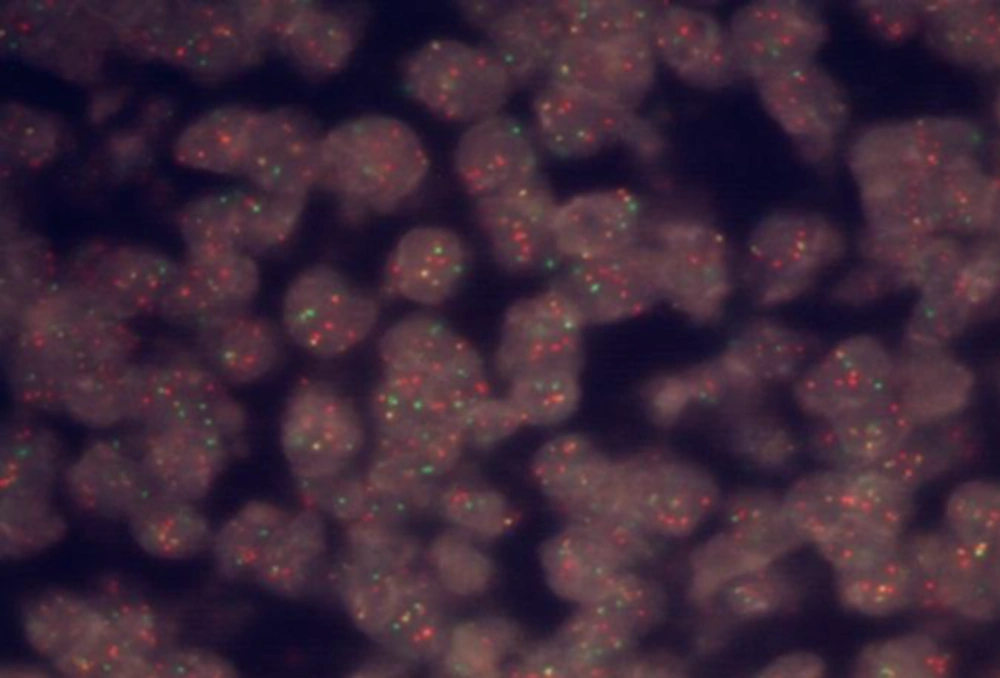
The histologic features and IHC findings recommended two differential diagnosis; pleomorphic MCL with loss of CD5 or Diffuse large B cell lymphoma with aberrant expression of Cyclin D1. Therefore for exact diagnosis, we performed fluorescence in situ hybridization (FISH) study for t (11; 14) (q 13; q 32) / CCND1 - IGH on paraffin embedded block at Dr Kariminejad-Najmabadi Pathology and Genetic Center (Tehran). The IGH (14q32) probe mix contains a proximal of IGH covering a region extending 600 kb, covering the genes JAG2, IGHC and IGHJ and a second probe covering a region extending 600 kb covering distal of the IGHV. Both are labeled in red. The BCL 1 (11q13) probe mix contains a proximal of BCL1 covering a region extending 510 kb, covering the genes MYEOV and CCND1 and a second probe covering a region extending 660 kb covering distal of the genes CCND1, FGF4 and FGF3. Both are labeled in green. The ratio of yellow fused signals to red ones is 43.9 % and the ratio of yellow fused signals to green ones is 54.6 %. The average is 49.2% which indicates CCND1 / IGH rearrangement in nearly all tumoral cells (Figure 2).
With proving CCND1 /IGH rearrangement by FISH test the diagnose of MCL was confirmed.
The right hemicolectomy specimen demonstrated no specific pathology findings with seven reactive lymph nodes in pericolic fat.
3. Discussion
Primary renal lymphoma accounts for 7% of all extra nodal lymphoma and represents less than 1% of all renal masses (4). By definition the term PRL is characterized by localized disease of the kidney without any sign of other organ involvement or when renal involvement is the presenting manifestation (2). Sign and symptoms contributing to PRL are various including flank pain, abdominal mass, weight loss, hematuria and acute renal failure in bilateral renal involvement. The most common reported PRL is diffuse large B cell lymphoma. Secondary MCL of the kidney has been reported before (5, 6) and few published studies have presented acute renal failure and renal function impairment associated with proliferative glomerulonephritis related to MCL (6, 7). In our case, no histologic evidence of glomerular lesion in remaining non-neoplastic tissue was seen. To our knowledge, primary renal MCL, pleomorphic variant has not been described before. MCL represented 5 - 10 % of all Non-Hodgkin Lymphomas. MCL afflicts male more than females and most commonly diagnosed in patients in their late 60s. The most probable sites of extra nodal MCL involvement are gastrointestinal tract followed by bone marrow, liver, spleen and Waldeyer’s ring while other sites are less infrequent (CNS, skin). Histology of typical MCL reveals monotonous small to medium sized lymphocytes with irregular or cleaved nuclei, condensed chromatin, indistinct nucleoli and scant cytoplasm forming diffuse, nodular or mantle zone patterns. Based on World Health Organization (WHO) classification, two aggressive variants of MCL are recognized: blastoid and pleomorphic. The blastoid variant is composed cells resemble lymphoblasts with dispersed chromatin and a high mitotic rate (usually at least 20 - 30/10 hpf). The pleomorphic variant is composed pleomorphic cells including many large cells with oval to irregular contour nuclei, often prominent nucleoli in at least some of the cells and generally pale cytoplasm (3).
IHC staining is used in diagnosis of MCL. MCL typically expresses B cell antigen (CD19, CD20, PAX5), CD5, Cyclin D1 (BCL1), BCL2, CD43 and is negative for CD10, CD23. Proliferation rate (Ki - 67) is a prognostic indicator and increases in aggressive variants of MCL. MCL with lack of CD5 expression has been observed in few studies and constitutes approximately 11 % of the cases (8); as depicted in our case. Lack of CD5 was not exclusively associated with any morphologic subtypes (8). Cyclin D1 which is a cell regulatory protein that aberrantly expressed in MCL as a result of the t (11; 14) (q13; q32) / CCND1 - IGH (9). Almost all cases of MCL are moderately to strongly positive for CyclinD1 and the neoplastic cells often show minor variation in staining intensity that most likely correlate with the phase of cell cycle (highest at G1 → S phase). In addition to MCL, cyclin D1 can be detected in a subset of cases of CLL / SLL, hairy cell leukemia and plasma cell myeloma. Also few studies showed Cyclin D1 expression by diffuse large B cell lymphoma (9). In this condition differentiation between diffuse large B cell lymphoma and aggressive MCL is difficult and molecular study for t (11;14) (q13; q32) / CCND1 - IGH is helpful.
Accurate diagnosis PRL is very important for different treatment options in comparison with renal cell carcinoma. Differentiating renal lymphoma from other renal masses is a diagnostic challenge, especially in cases of unilateral lesions since they simulate renal carcinomas in radiologic evaluation. So renal lesions that lack typical radiological features of renal cell carcinoma or multinodular involvement of the kidney along with lymphadenopathy merits a biopsy (10). Generally the standard management of a renal mass is nephrectomy. However, systemic chemotherapy with or without radiotherapy is currently the first treatment option for PRL (11). Although most authors believe that the CHOP protocol should be an elective option (as it is in Non-Hodgkin B cell Lymphoma). There is no agreement upon standard treatment approach (es) for PRL (12). Long term survival occasionally has been reported after combination of surgery with chemotherapy in case of unilateral involvement of kidney by PRL (13). However, patients with aggressive variant of MCL are associated with particularly short durations of response after chemotherapy and poorer overall survival (14).
Unfortunately in our case due to continuation of treatment in another clinical center, no exact data for chemotherapy regimen is available. Nevertheless this patient is alive after one year from initial diagnose.