1. Background
Wilms’ tumors are the most common cancers in children that are diagnosed in the kidneys, with a general cure rate of about 85%, using recommended strategies for management. The outstanding results were the consequence of close cooperation between surgeons, oncologists, and pathologists. So, a multidisciplinary approach leads to reasonable results in children with Wilms’ tumors. But, there are many challenges in identifying novel treatment intensity for longer survival (1). Progress in the multimodality management of cases with Wilms tumor has shown appreciably enhanced survival in this group. Unfortunately, there are no sufficient studies on survivors of childhood cancer after achievement to young adults. They are at risk for developing chronic health problems. Generally, late complications are the result of management type and kind of outcome evaluation. The employment of nephrectomy followed adjuvant therapy with vincristine and actinomycin in low-stage illness has demonstrated at least late effects. Dissimilarity, cases with higher-stage or relapsed sickness frequently need radiotherapy, raising the risk of late complications.
2. Objectives
This study assesses the individualities of Wilms’ tumor and description of the results of management in cases operated through 2 decades in our center. As there was a gap between data gathering in our center, we decided to report these patients in two decades to find a way to improve the survival of these children.
3. Methods
3.1. Patients
This review was performed for all patients, who underwent an operation for Wilms’ tumor at the Mofid Hospital from 1992 to 2002 (2) and 2006 through 2016. There was a gap between these two decades due to data loss.
All the cases with a pathologically confirmed the recognition of Wilms’ tumor integrated with this report. Patients who underwent surgery or adjuvant therapy in another center were excluded.
All hospital files of children with Wilms’ tumor were analyzed for sex, age at the operation, the form of appearance, side of the concerned kidney, related underlying disease, preoperative and postoperative management, type of operation, stage of the disease, rate of relapse and metastasis, and results of follow-up.
In decades, clinical staging and histopathologic classification were evaluated base on Children’s Oncology Group (COG) Wilms’ Tumor.
3.2. Patient’s Assessment
In the first decade, ultrasonography of the kidney, abdominal computerized tomography (CT), radiography of chest, and intravenous pyelography (in initial patients) were employed to inspect the children. Ultrasonography and abdominal CT scan were identified as the maximum beneficial and precise methods for the assessment of the cases previous to surgery. In the second decade, a CT scan of thorax was done for 70% of patients and Doppler ultrasound was done in 6.7% of patients; 11.1 % of patients underwent bone marrow aspiration and bone survey. One patient underwent a brain CT scan that was normal.
In both decades, relapse and metastasis of Wilms’ tumor were detected by abdominal ultrasound and chest x-ray that was performed every 3 months for the first 2 years after operation and then requested every 4 to 6 months in the next third and fourth year and annually in the fifth year. CT scans of the chest, or abdomen and sometimes abdominal MRI were done when the initial investigation was abnormal.
3.3. Patient’s Treatment Strategies
In both decades in unilateral form, radical nephrectomy was performed at first and then underwent chemotherapy. Only in bilateral tumor and extensive involvement, they underwent preoperative chemotherapy after the incisional biopsy.
3.4. Analyzed Factors and Statistical Analysis
We used files and detailed questionnaires to collect information. All collected information was statistically analyzed by SPSS software version 17. Discrete variables were reported as numbers and percentages. All continuous variables were mean and standard deviation. Demographics, preoperative data, operative details, and early postoperative complications were expressed as means, standard deviation, or median and ranges. The Kaplan-Meier method was obtained for survival rate.
3.5. Ethical Considerations
This study was accepted by the Institutional Board Review and the Medical Ethics Committee of our University of Shahid Beheshti University Medical Sciences, with the ethical committee code of SBMU.REC.1392.718. No additional intervention or cost was imposed on patients in this study. Patients’ data entered into the study as an encoded parameter without mentioning the name of any participant. The personal information of none of the patients was included in this research, and just their statistical analysis was presented in general. The informed consent was obtained from the legal guardian(s) of each participant in the study; nevertheless, patients were excluded from the study in cases, where the patients or their parents did not consent to participate in the study
4. Results
In the first decade, 55 patients were identified with Wilms’ tumor in Mofid Children’s Hospital. Of them, 24 (45.2%) were females and 31 (56.3 %) were males (M/F = 1.29). In the second decade, 60 patients were operated as Willms’ tumor in Mofid Hospital, and pathology was verified but in 11 patients, adjuvant therapy was performed in another center; so, they were excluded from our study. In 49 patients, 19 (38.8%) were males, and 30 (61.2%) were females (M/F = 0.61). In the first decade, the mean age at operation time was 45.2 months ranged between 2 to 120 months; in the other group, the mean age at the time of operation was 36.2 months ranged between 1 to 120 months.
Table 1 shows the associated underlying disease in both decades. Some cases were identified with more than one anomaly.
| Associated Underlying Disease | Number of Patients in the First Decade | Number of Patients in the Second Decade |
|---|---|---|
| Hypospadias | 3 | 1 |
| Undescended testis | 3 | 3 |
| Congenital cataracts | 2 | . |
| Aniridia | 1 | . |
| Disorder of sexual development | 1 | . |
| Ureter duplication | 1 | . |
| Cleft lip | 1 | . |
| Inguinal hernia on both sides | 1 | 1 |
| Down’s syndrome associated with VSD and ASD | 1 | . |
Associated Underlying Disease with Wilms’ Tumor
In both groups, the majority of presentations at the time of admission were abdominal enlargement and mass recognized by the physicians or parents. Table 2 shows forms of initial presentation. Some children were presented with more than one symptom.
| Initial Symptom and Sign | Percentage in the First Decade (55 Patients) | Percentage in the Second Decade (60 Patients) |
|---|---|---|
| Palpable abdominal mass and abdominal enlargement | 90.9% (50) | 55% (33) |
| Hematuria in urine | 14.5% (8) | 16.7% (10) |
| Acute abdomen | 10.9% (6) | 15% (9) |
| Fever | 5.5% (3) | 10% (6) |
| Weight loss | 1.8% (1) | 0 |
| Diarrhea | 1.8% (1) | 0 |
| Abdominal bleeding secondary to tumor rupture | 0 | 3.3% (2) |
| Urinary disturbance | 0 | 1.6% (1) |
| Prenatal diagnosis | 0 | 3.3% (2) |
Form of the Initial Appearance of Wilms’ Tumor
In the first group, the left kidney was involved in 30 cases (54.5%). One patient demonstrated bilateral form. In the second group, the left kidney was involved in 23 (46.9 %) patients, and the right one in 21 (42.9%) patients; 5 patients (10.2%) had bilateral Wilms’ tumor at presentation.
In the first group in two patients, the tumor was extensive; so, only tumor biopsy was performed, one presented with Budd-Chiari syndrome and another with skull metastasis at the first admission. They underwent preoperative chemotherapy after the incisional biopsy. One case with bilateral Wilms’ tumor was managed with radical nephrectomy on the left side and incomplete nephrectomy on the right side. In 2 cases, the right renal vein and inferior vena cava (IVC) were occupied. The ureter was involved in one case.
In the second decade in the unilateral tumor, preoperative chemotherapy was done in 2 cases after obtaining biopsies (Fine needle aspiration in one case); in other cases, nephrectomy was done in the first session. Three patients with bilateral Wilms’ tumor underwent radical nephrectomy on one side and incomplete nephrectomy on the other side. However, 2 cases at the first surgery underwent biopsy from both sides; then, partial nephrectomy was performed after chemotherapy. A segment of the diaphragm was removed in one case associated with resection of the tail of the pancreas. Routine lymph node sampling was done in 60% of patients; 6.1% of patients had tumor spillage during surgery. Segmental colectomy was performed in one case. One patient underwent splenectomy because of spleen bleeding; 8.2% of the patients underwent radical adrenalectomy.
Pulmonary nodules were seen in 28.6% of patients and 10.2% appeared before surgery; 2 (3.6%) cases of IVC were concerned and the renal vein was occupied in 3 patients. Some patients had more than one presenting intraoperative finding.
4.1. Surgical Stage
Table 3 shows the stages of disease in patients and demonstrates the distribution of stages along with sexes. In the first decade, 7 complications needed admission. In two cases, myelosuppression occurred (the occurrence of pancytopenia, epistaxis, petechia, and sepsis owing to neutropenia). Two convulsions episode occurred after chemotherapy. Vincristine led to peripheral neuropathy in one child. Renal failure occurred in one case with unilateral Wilms’ tumor. Hepatitis secondary to chemotherapy happened in one case. In the second decade, one patient expired during surgery from IVC involvement and intraoperative emboli. One patient had obstruction after the first surgery and renal failure was seen in one patient.
| Surgical Stage | |||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| First decade Number intraoperative staging (%) | 18 (F = 5) 33% | 9 (F = 8) 16% | 19 (F = 9) 38% | 5 (F = 2) 9% | 1 (F = 0) 1.8% |
| Second decade Number intraoperative staging (%) | 7 (F = 5) 14.3% | 20 (F = 13) 40.7% | 12 (F = 6) 24.5% | 5 (F = 3) 10.2% | 5 (F = 3) 10.2% |
Separation of Intraoperative Stages Between Two Decades and Distribution of Stages Along with Sexes
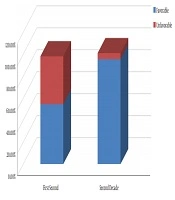
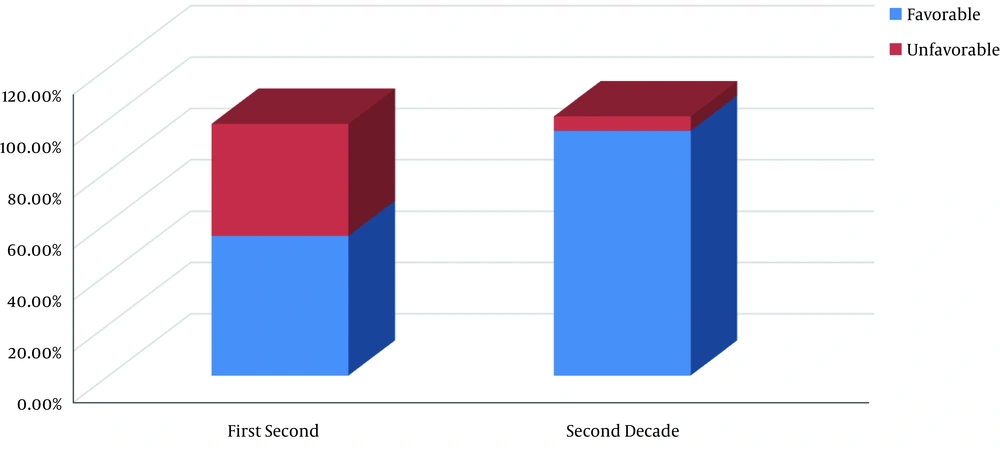
Favorable histology was found in the first decade in 30 (54.5%) patients and unfavorable histology was found in 24 (43.6%) cases (Figure 1). A total of 20% of patients with unfavorable histology was recorded as 7.3% anaplasia (focal or diffuse) and 12.7% clear cell sarcoma. In the second decade, 95.4% were the favorable type (Figure 1).
In the first decade, tumor relapse was detected in 4 cases (7%), 3 patients were stage III, and 1 patient was stage IV. Histologic findings in 4 cases included 3 unfavorable histology and one favorable type. Places of relapse were detected in the abdomen (3 patients) and pulmonary (1 patient). One case reported recurrence in the abdominal cavity secondary to surgical spillage of the mass about 13 months after the primary operation. One case was identified with a metachronous bilateral Wilms’ tumor. The places of metastases were pulmonary (3 patients), brain (1 patient), and liver (3 patients).
In the second decade, one patient expired; 90% of patients were cured for more than 4 years and 2.3% had liver metastasis. Pulmonary metastasis was seen in 28.6% and recurrent tumors were seen in 5.7% of cases.
4.2. Survival
In the first decade, the relative relapse-free occurred in 71% of cases, the overall 4-year survival rate was 86%, and for the second decade, the 4-year survival rate was 90%.
Respectively in the second group, we need extended follow-up. It was not easy to describe the long-standing survival rate of each stage discretely, as there were few cases in each stage.
Some causes of mortality included operation of skull metastases, hepatitis due to some drugs, IVC involvement, lung emboli, and Down’s syndrome coupled with cardiac diseases.
5. Discussion
The prognosis of patients with Wilms’ tumor was noticeably enhanced compared to the previous 3 decades. International Society of Pediatric Oncology (SIOP) and the National Wilms Tumor Study Group (NWTSG) carried out variant reports for therapy of a patient with Wilms’ tumor (3).
The 4-year survival rate describes the percentage of patients, who are alive at least 4 years after their cancer is identified. The most important aspects in establishing a child’s outlook are the degree of stage and histology type of the tumor. In favorable histology, there is no anaplasia and more than 90% of tumors contain favorable histology. The possibility of healing children with these tumors is outstanding. In unfavorable histology (anaplastic form), the appearance of the cancer cells show discrepancy extensively, and the cells’ nuclei tend to be very large and distorted. With the tumor with more anaplasia, the chance of cure decreases.
In this study, in the first decade, the majority of presenting signs included abdominal mass (90.9%). But, in the second decade, it reached 54.2%. The reason that the abdominal mass was reported as the majority sign in the first decade can be followed higher stages of the disease in our cases at the first appearance in the last.
The surgical stages of our cases were compared to National Wilms’ Tumor study (NWTS): stage I patients comprised 42% of all patients in NWTS3, while in this study it was found in 32.7% of patients in the first decade and 14.3% in the second decade. Also, the rate of the bilateral tumor was 5 times higher in the second decade (4-6). Thus in Iran, sick children were submitted to specialized centers with much delay.
Unfavorable histology was reported in nearly 10% of childhood with renal masses. It is uncommon in ages before 2 years old (2%) and, then, is amplified in cases more than 5 years to 13% (7). It observes more common in nonwhite than in white patients. In our study, in the first decade, 54.5% of our cases were presented with favorable and 43.6% showed unfavorable type.
The amount of unfavorable category in NWTS3 showed about 11.12% (8-10). This might propose the existence of extra violent appearance of the illness in our area, or it can owe to postpone in the appearance by few numbers of our cases. This information entitles for the arrangement of a comprehensive study in this area of the world. Arrangement of this group would direct to intensive cases in addition to a supplementary methodical approach to the management and create suitable protocols for the children. But, in a recent study on the second decade, the rate of unfavorable histology was 5.6% and this difference is significant.
A suitable surgical approach is important for risk-based treatment. Wrong or imperfect actions lead the cases to additional therapy with possible long-standing toxicities of adjuvant therapy. Ehrlich et al. (11) assessed the characteristics of surgical protocol violations (SPV) in Wilms tumor and SPV was described as any difference from the international protocol and built-in lack of any lymph node sampling, an inaccurate abdominal incision, intraoperative spillage, needless resection of organs, and biopsy without delay before nephrectomy. Very low risk (VLR) Wilms tumor is described as favorable histology, tumor weight less than 550 grams, age less than 2 years, and stage I. Currently, COG advocates nephrectomy for these children (12-14). Survival rates of low-stage tumors are about 95% to 99%. In this study, in the first and second decades, 32.7% and 14% of them, respectively, were in stage I. But, all of them underwent radical nephrectomy and adjutant therapy and the survival rate for low-stage patients was 100%.
Metastatic disease (stage IV) was documented as an unlucky prognostic factor for patients with Wilms’ tumor (15). New articles report that the involvement in the liver as the first sign in patients with Wilms’ tumor leads to an inferior prognosis than lung or other locations of stage IV sickness (16-18). In this study, in both groups, the majority of presentations at the time of admission were abdominal enlargement and mass recognized by the physicians or parents. Liver involvement was not the first sign in any patients. Unlike other investigations, Ehrlich et al. (19) studied 742 patients with stage IV Wilms’ tumor, 111 of whom had liver metastases. It was reported that liver involvement at the first presentation is not an unfavorable prognostic issue for stage IV. In this study, in the first group, the lungs were presented as the majority location of distant metastases (43%), and the next organ was reported liver. In the second group, pulmonary metastasis was noticed in 28.6% and liver involvement in 2.3 %. Irtan et al. (20) reported that biopsy or preoperative chemotherapy is useful for the management of the patient with disease stage III. In chosen cases, with tumor stage, III WT can stay alive with no radiotherapy (20).
Doganis et al. (21) stated that despite the excellent overall prognosis in children with Wilm’s tumor except for children patients with advanced or bilateral disease and/or high-risk histology still experience unfortunate outcomes (21, 22). Our study also showed the same consequences.
Few reports for protocols and management results of Iran are available. Keeping away from surgical spillage of cancer and putting emphasis on the position sampling of lymph nodes will decrease the hazard of recurrences. Consideration to address to world management protocols, the recommendation to early identification of involved patients by ultrasonography, and diminish the number of cases missing to follow-up, the most favorable consequences look to be reached. A bigger sample size would supply superior assurance to the universal judgment of these consequences. We advocate more complete studies with particular notice to long-standing follow-up of cases for learning the late side effects of management and recognize survival rates in every phase and histopathologic grouping.
5.1. Conclusions
The outstanding results have been the consequence of close cooperation between surgeons, oncologists, and pathologists. So, a multidisciplinary approach leads to reasonable results in children with Wilms’ tumors. In this study, in the second decade, surveillance increased; so, with more adjustment in treatment protocols, the superior outcome will be attainable.