1. Background
Acute graft versus host disease (aGVHD) following hematopoietic stem cell transplantation (HSCT) is an immune and inflammatory response that happens when donor T cells respond to the incompatible proteins on the host cells (1). Development of aGVHD includes three sequential phases: activation of innate immune cells after chemoradiotherapy conditioning regimen; donor T-cell activation, proliferation, and differentiation; T cells migration to target tissues; and finally tissue damage (2). On the other hand, immune cells such as monocytes, dendritic cells, and T cells are considered as important players during the aGVHD initiation (3, 4).
Long non-coding RNAs (lnc-RNAs) classified as regulatory transcripts with more than 200 nucleotides in length (5). These noncoding RNA molecules exist in the blood circulation, as well as different tissues and body fluids (6). Their significant regulatory function has been demonstrated in various cellular pathways such as cycle cell, apoptosis, autophagy, and cell signaling (7, 8). Recently, it was also reported that few lnc-RNAs can regulate gene expression in immune and inflammatory responses (9). They are fundamental regulators of gene expression in the immune cells which control different biological functions including cell differentiation, proliferation, activation, cytokine secretion, and cell migration. They stimulate the transcription of inflammatory cytokines and their target genes (10). In addition, they are emerging as biomarkers for diagnosis, prognosis, and therapeutic targets of various diseases especially cancers (11).
Studies have shown that lncRNAs are frequently deregulated in many immune disorders such as hashimoto’s thyroiditis (HT) and ulcerative colitis (UC) with strong association with the pathogenesis and outcome of these disorders (12). As a result, identification of Lnc-RNAs in various diseases can lead to a better diagnosis, prognosis, and possibly discovering novel therapeutic options (13).
Lnc-DC is an intergenic lncRNA that plays an important role during differentiation of human monocytes into dendritic cells, as well as T cell activation (14). Studies also demonstrated that the knockdown of lnc-DC can decrease the expression of several genes such as CD40, CD80, CD86, and HLA-DR, resulting in impaired antigen uptake by antigen presenting cells (APCs), reduced allogenic T CD4+ cell activity, and attenuated cytokine release (15). Therefore, the suppression of lnc-DC can inhibit the antigen presentation and T cell response in immune disorders (16).
2. Objectives
With regard to the importance of interaction between donor T cells and recipient APC in aGVHD pathogenesis and the role of lnc-DC in immune responses (17), this study was aimed at investigating the expression levels of lnc-DC in the aGVHD development.
3. Methods
3.1. Subjects
A total of 50 patients with hematologic malignancies primarily participated in this study among which 12 patients were excluded according to exclusion criteria. Therefore, we included 38 patients (15 females and 23 males, mean age = 42.8 years) with acute myeloid leukemia (AML) (n = 23) and Acute lymphocytic leukemia (ALL) (n = 15) who underwent primary allogenic bone marrow transplantation at Taleghani Hospital of Tehran between September 2017 and June 2018.
Patients with aGVHD and chronic Graft-versus-Host disease (cGVHD) were diagnosed based on consensus criteria of National Institute of Health (NIH). The aGVHD grading was determined by its overall severity based on clinical impressions observed in the skin, rectal, stomach, or duodenal biopsies. Allogenic hematopoietic stem cell transplantation (HSCT) was obtained from antigen-identical siblings or unrelated donors. The alternative diagnosis such as infection, disease relapse, and drug toxicity were considered as exclusion criteria. Informed consent forms were filled by all patients before transplantation and the study was approved by the Medical Ethics Committee of Shahid Beheshti University of Medical Sciences (ethical code: IR.SBMU.RETECH.REC.1369.798)
The characteristics of patients, including demographic and clinical data are summarized in Table 1.
| Characteristic | GVHD | Non-GVHD | Significance Level | ||
|---|---|---|---|---|---|
| P Value Summery | Odd Ratio | 95% Confidence Interval | |||
| Age1 (range) | 28 - 45 | 22 - 60 | |||
| Missing | 0 | ||||
| Sex | |||||
| Female | 6 (46.1) | 10 (40.0) | 0.3915, ns | 1.278 | 0.7291 - 2.239 |
| Male | 7 (53.9) | 15 (60.0) | |||
| Missing | 0 | ||||
| Diagnosis | |||||
| AML | 9 (69.2) | 14 (56.0) | 0.0576, ns | 1.749 | 0.9798 - 3.122 |
| ALL | 4 (30.8) | 11 (44.0) | |||
| Missing | |||||
| Conditioning regimen | |||||
| Bu/Cy | 8 (61.5) | 12 (48.0) | 0.0649 | 1.694 | 0.9662 - 2.972 |
| Bu/Fu | 3 (23.0) | 9 (36.0) | |||
| Bu/Fu/ATG | 2 (15.3) | 4 (16.0) | |||
| Missing | 0 (0.0) | 0 (0.0) | |||
| Donor-recipient relationship | |||||
| Sibling | 11 (84.6) | 22 (88.0) | 0.5348 | 0.7727 | 0.3418 - 1.747 |
| Non-sibling | 2 (15.4) | 3 (12.0) | |||
| Missing | 0 (0.0) | 0 (0.0) | |||
| HLA matching A, B, DRB1(10/10) | |||||
| Yes | 13 (100.0) | 23 (92.0) | 0.0039** | 18.47 | 1.051 - 324.7 |
| No | 0 (0.0) | 2 (8.0) | |||
| Missing | |||||
| GVHD prophylaxis | |||||
| CSA + MTX | 8 (61.5) | 14 (56.0) | 0.4730 | 1.229 | 0.6995 - 2.159 |
| CSA + MTX + ATG | 5 (38.5) | 11 (44.0) | |||
| Missing | |||||
Abbreviations: AML, acute myeloid leukemia; ATG, anti-thymocyte globulin; Bu, Busulfan; CSA, cyclosporin A; Cy, cyclophosphamide; Fu, fludarabine; GVHD, graft versus host disease; MTX, methotrexate.
aValues are expressed as No. (%).
3.2. Sample Collection
Stem cells for transplantation were isolated from peripheral blood and the patients received non-T cell-depleted graft. All the blood samples were obtained from HSCT recipients at days 0, 7, 14 and 28 and the final day after transplantation. PBMCs were isolated from all samples by Ficoll-Hypaque gradient (density, 1.077), followed by centrifugation at 400 × g for 30 min. The buffy coats were collected and washed twice with phosphate-buffered saline (pH = 7.4).
3.3. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
Total RNA was isolated from mononuclear cells of the patients using the TRIzol reagent (GeneAll‐RiboEx LS Total RNA Solution) according to the manufacturer's protocol. The RNA samples were treated with RNase-free recombinant DNase I (Roche, Mannheim, Germany). The cDNA was synthesized from total RNA using the Prime Script RT reagent Kit (perfect real time) (TAKARA BIO INC.) according to the manufacturer’s instructions. We used the GENE RUNNER software to design the Gene-specific primer. The primer sequences were as follows:
Lnc-DC:
Sense: 5’-TTTTGGGTACCTGCGTCGAG-3’.
Antisense: 5’-CCTGTCCTTACCCTGCAACA-3’.
ABL:
Sense: 5’-CTTCTTGGTGCGTGAGAGTGAG-3’.
Antisense: 5’-GACGTAGAGCTTGCCATCAGAAG-3’.
The qRT-PCR reaction was performed to assess lnc-DC gene expression using the RealQ Plus × 2 Master Mix, green (high ROX) (AMPLIQON, Odense M, Denmark) using an Applied Biosystems StepOnePlusTM instrument. The PCR amplification conditions were as follows: the first denaturation at 95°C for 10 min, 40 cycles of denaturation at 95°C for 15 sec, annealing at 60°C for 20sec, and a final extension for 15sec at 72°C. The gene expression level was measured as the ratio to the ABL transcript level. The results were then analyzed using the Bio-Rad CFX Manager software.
3.4. Statistical Analysis
All data are shown as mean ± standard error of the mean (SEM) values. One-way analysis of variance (ANOVA) was to analyze more than 2 groups. The difference in the frequency of each gene between recipients at the different time intervals was assessed using a two-tailed Wilcoxon matched-pairs signed-rank t-test. The P values of less than 0.05 were considered as statistically significant. All the analyses were performed by using the GraphPad Prism 6 software (version 6.0.3; GraphPad Software Inc., San Diego, CA, USA).
4. Results
4.1. Clinical Information of aGVHD and Non-GVHD Patients
The mean age of aGVHD and non-GVHD cases was 37.2 ± 9 and 41.6 ± 20.1 years, respectively. There were 6 (46.1%) females and 7 (53.9%) males in the aGVHD group, and 10 (40.0%) females and 15 (60.0%) males in the non-GVHD group (P = 0.3915). The detailed clinical and pathological characteristics of aGVHD and non-GVHD cases are indicated in Table 1.
Among 38 patients who underwent BMT, 7 patients indicated skin aGVHD manifestations, 5 patients showed gastrointestinal aGVHD symptoms, and 1 patient had liver aGVHD signs. Due to the low number of bone marrow transplanted patients involving aGVHD, the statistical population in this study was limited and thus calculated with the minimum population based on the Charles Cochran standard.
4.2. Expression Analysis for LncRNA-DC
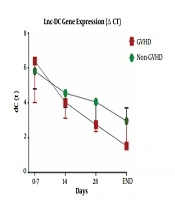
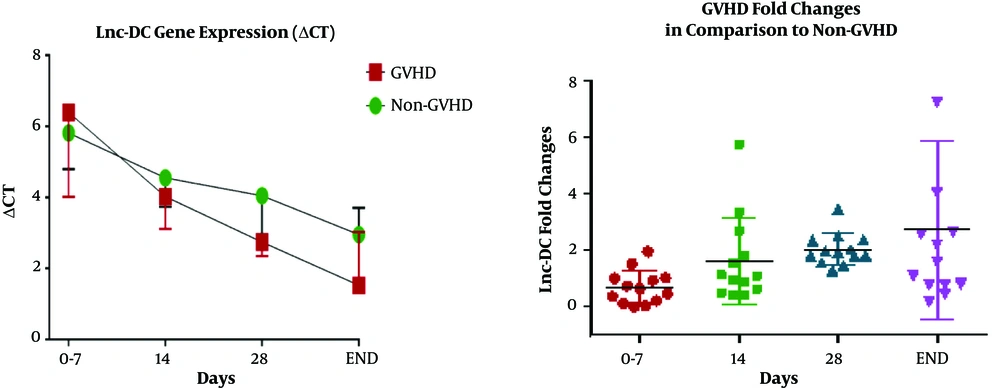
Lnc-DC gene expression level between patients who underwent BMT with aGVHD and non-GVHD on days 0, 7, 14, 28, and the final day were compared. Final days were 52 ± 8 days after transplantation for those who experienced aGVHD, and 60 ± 9 days for those without aGVHD. As expected, the ΔCT of the lnc-DC gene between the two groups of aGVHD and non-GVHD showed no significant statistical differences (P = 0.3) up to 14 days after transplantation. However, the lnc-DC level was significantly upregulated in patients with aGVHD on day 28 (P = 0.03). The mean ΔCT in aGVHD and non-GVHD groups was 2/796 ± 0/1395 and 4/043 ± 0/3960, respectively. Furthermore, the follow-up studies on these two transplant recipient groups revealed that in addition to the relative increase in lnc-DC gene expression in aGVHD group, there was still an interesting association between the incidence of aGVHD and the increased gene expression (P = 0.01). The lnc-DC was upregulated at the onset of aGVHD manifestations compared with non-GVHD patients (2.6-fold change). The relative expression data are shown in Figure 1 and Table 2.
| Patients Status | Mean ± SEM | Difference Between Means | 95% Confidence Interval | P Value | P Value Summary | Fold Changes |
|---|---|---|---|---|---|---|
| Day 0-7 | -0.7311 ± 0.7668 | -2.286 - 0.8240 | 0.3360 | ns | 0.602 | |
| GVHD (n = 13) | 6.542 ± 0.7211 | |||||
| non-GVHD (n = 25) | 5.811 ± 0.4081 | |||||
| Day 14 | 0.4747 ± 0.5150 | -0.5698 - 1.519 | 0.3628 | ns | 1.389 | |
| GVHD (n = 13) | 4.056 ± 0.4054 | |||||
| non-GVHD (n = 25) | 4.531 ± 0.3056 | |||||
| Day 28 | 1.247 ± 0.5616 | 0.1078 - 2.386 | 0.0328 | * | 2.373 | |
| GVHD (n = 13) | 2.796 ± 0.1395 | |||||
| non-GVHD (n = 25) | 4.043 ± 0.3960 | |||||
| END day | 1.383 ± 0.5403 | 0.2876 - 2.479 | 0.0148 | * | 2.608 | |
| GVHD (n = 13) | 1.579 ± 0.4505 | |||||
| non-GVHD (n = 25) | 2.962 ± 0.3115 |
Abbreviation: GVHD, graft versus host disease; Ns, non-significant.
4.3. Evaluation of the Diagnostic Value of Lnc-DC in aGVHD
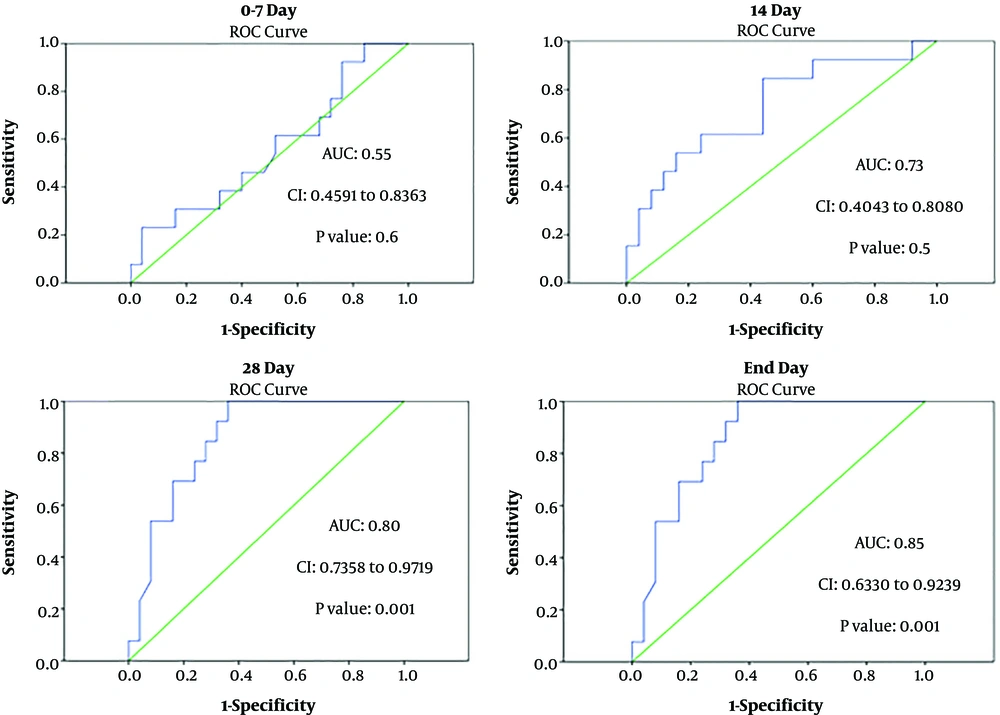
According to the receiver operating characteristic (ROC) curve analysis, the lnc-DC gene expression had an acceptable total area under the curve (AUC) on the days studied. AUC was reported to be greater than 0.7 up to 14 days and greater than 0.8 from day 28 to the final day. On the other hand, ROC curve analysis showed that the sensitivity and specificity of this gene are acceptable for discriminating between aGVHD and non-GVHD individuals from day 28 onwards. The ROC analysis results are displayed in Figures 2.
5. Discussion
Acute GVHD remaines a major complication after allogeneic HSCT that can affect the outcome and immune reconstitution of bone marrow transplantation. Despite all of the aGVHD prophylaxis and immunosuppressive therapies, it can occur in approximately 35% to 70% of HSCT recipients (18).
aGVHD is an immune disorder and its abnormalities initiate with APCs and immune cells activation, releasing the proinflammatory cytokines, and T-cells responses (19). The aGVHD detection is a challenging effort, which is usually diagnosed when patients demonstrate clinical symptoms (20). As a result, the diagnosis of high-risk patients is important and may facilitate managing and modifying treatment approaches in susceptible patients and prevent the onset of severe symptoms and death (18). A better understanding of molecular mechanisms and genes involved in aGVHD pathophysiology can improve our insights into aGVHD pathogenesis, diagnosis, and targeted therapies to inhibit aGVHD. In addition, more specific biomarkers are needed for the objective diagnosis and for predicting the aGVHD onset (20). In previous studies, noncoding RNAs especially miRNAs were proven as potential biomarkers for diagnosis, prognosis, and prediction of aGVHD (21). Altered levels of miRNAs are involved in the development of aGVHD and can be used for aGVHD prediction.
Recent studies indicated that microRNAs that are involved in the regulation of immune system, differentiation, and activation of the immune cells were significantly associated with aGVHD incidence before the disease onset (median at day +28 after transplantation) (22). However, there are no reports about aberrant changes in the expression of LncRNAs in aGVHD development so far (23).
Similar to the miRNAs, lncRNAs are transcripts that have important roles in the differentiation and function of immune cells. Wang et al. revealed that lnc-DC was exclusively expressed in monocytes and dendritic cells, the most potent APCs of the immune system (24). Lnc-DC interacts with signal transducer and activator of transcription 3 (STAT3) and then stimulates its phosphorylation and activation (25). The role of lnc-DC in the regulation of STAT3 signaling was recently revealed in coronary artery disease and type 2 diabetes mellitus. They also showed that the promoter region of lncDC contains a binding site for PU.1, a key regulator of monocyte and dendritic cell differentiation (26). These results indicated that lnc-DC has important role in monocytes/dendritic cells differentiation.
Wang et al. (25) also indicated that the knockdown of the lncDC leads to the downregulation of cell surface molecules such as CD40, CD80, CD86, and HLA-DR, which are important for T cell activation. This gave rise to impaired antigen uptake by APCs and allogeneic CD4-positive T cell proliferation and cytokine release (27). With regard to previous studies, Zhang et al. (28) also indicated that overexpression of lnc-DC can lead to imbalance of immune responses and increased Th1 cells in preeclampsia patients.
Studies by Zhuang indicated that the level of lnc-DC expression in human monocytes and dendritic cells was significantly associated with activation of TLR/STAT3 signaling, dendritic cell proliferation, and strong immune response (29). Consequently, in this study, we investigated the expression level of lnc-DC in aGVHD development (14).Our findings showed that lnc-DC expression in the aGVHD patients was higher than non-GVHD patients (P < 0.01), and also confirmed that patients who had a higher lnc-DC expression level in peripheral blood on day 28 after transplantation were at a higher risk of developing aGVHD. As a result, there may be a relationship between the expression level of lnc-DC and the timing of aGVHD occurrence. It was already reported that the levels of lnc-DC were significantly higher in patients with lupus nephritis (LN) compared with SLE without nephritis. As a result, Lnc-DC in plasma could be a potential biomarker for distinguishing the LN from SLE without nephritis (30).
Shaker et al. (31) also showed that blood levels of lnc-DC were increased in patients with multiple sclerosis (MS). Moreover, there was a positive correlation between lnc-DC expression and relapse occurrence in patients with MS. They indicated by ROC curve analysis that lnc-DC had 100% specificity and 64.4% sensitivity for MS diagnosis, suggesting the lnc-DC as a promising marker for diagnosis of patients with MS. In the current study, the ROC curve analysis also confirmed that the lnc-DC had valuable AUC for aGVHD prediction on day 28 of transplantation.
5.1. Conclusions
Results of the present study indicated that the lnc-DC might involve in developing aGVHD and could be considered as a novel marker for aGVHD prediction, although further studies with a larger sample size are necessary to validate these results.