1. Background
Cancer-induced cachexia is defined as one of the most common syndromes in patients with advanced malignancies and is associated with the quality of life and survival impairment (1). According to the 2014 estimation, the prevalence of cancer-induced cachexia is about 50% to 80% in patients with cancer and accounts for up to 20% of cancer-related deaths (2). The latest definition of cancer-induced cachexia by international consensus group is “a multifactorial syndrome by an ongoing loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and leads to progressive functional impairment” (3).
Cachexia is a complex syndrome related to underlying chronic diseases condition such as cancer, acquired immunodeficiency syndrome (AIDS), rheumatoid arthritis, chronic infection, and chronic obstructive pulmonary disease (4). Common symptoms of cachexia are anorexia or reduced nutritional intake, a systemic inflammatory response, fatigue, and decreased muscle strength (3, 5). The most probable mechanism suggested by in vitro and in vivo studies is correlated with inflammatory cytokine activation (tumor necrosis factor-alpha [TNF-α], interleukin IL-1, and IL-6) and several tumor-derived substances, which lead to skeletal muscle destruction (6, 7).
The management of cancer-induced cachexia is pivotal. Many therapeutic modalities have been suggested in preclinical and clinical studies for the management of cancer-induced cachexia; however, only a small number of drugs such as megestrol acetate, anabolic steroids, and glucocorticoids are used extensively (8-11). On the other hand, numerous therapeutic agents including omega-3, carnitine supplement, nonsteroidal anti-inflammatory drugs (NSAIDs), and thalidomide aiming to improve the patients’ cachexia are under investigation (12-15). Along with all efforts, there is no documented agent for the management of cancer-induced cachexia. In some European countries and the United States, megestrol acetate was approved for the management of cancer-induced cachexia and anorexia (9).
Herbal medicines were welcomed in decades for the management of diseases and its complication. Today, the therapeutic mechanisms and their active compounds have been gradually uncovered. For example, encouraging results have been released by the anti-inflammatory effects of Fenugreek and Chicory in animal studies. The proposed anti-inflammatory mechanisms of both herbal components are a positive impact on inflammatory cytokines (TNF-α, IL-1, and 6) reduction (16, 17). Theoretically, this mechanism can be considered for the prevention and management of cancer-induced cachexia.
2. Objectives
Furthermore, in this study, by considering the possible effect of some herbal combination on ameliorating inflammatory cytokine, we selected Fennel (Foeniculum vulgare), Fenugreek (Trigonella Foenum-graecum), and Chicory (Cichorium intybus) as a possible effective traditional herbal combination and aimed at evaluating the possible protective effect of them in the management of cancer-induced cachexia/anorexia in patients suffering from cancer.
3. Methods
3.1. Study Design and Patients Recruitment
The present study was a randomized, double-blinded, placebo-controlled trial carried out from July 2017 to June 2019 at a referral and tertiary hematology-oncology center in Omid Hospital (affiliated to Isfahan University of Medical Sciences), Isfahan, Iran. The study was conducted on an 8-week follow-up period on patients suffering from cachexia and anorexia due to advanced solid tumors. The Ethics Committee of Isfahan University of Medical Sciences approved the study. Clinical trial registration was IRCT20180722040556N2 (Iranian Clinical Trials Information) and all patients signed the written informed consent.
The inclusion criteria were adult patients (18 years old) with advanced solid tumors (stage 3 - 4), who had weight-loss equal or greater than 5% within 2 months and managed by a high dose of megestrol (160 mg/day) as a routine cachexia therapy. We also considered other inclusion criteria such as no evidence of gastrointestinal obstruction, having serum creatinine more than 2 mg/dL, and total bilirubin less than 2 mg/dL, not any use of steroids products, and no history of any chronic disease except cancer. The included patients were expected to have 3 months of life expectancy based on clinical judgment.
Patients were excluded if they had major surgery in the last month, brain metastasis and history of diseases such as thrombophlebitis, uncontrolled diabetes or blood pressure, heart failure, and cognitive or psychiatric disorders. Moreover, pregnant and lactating mothers and patients with known sensitivity to one of the included plants in the herbal combination were not enrolled.
3.2. Intervention
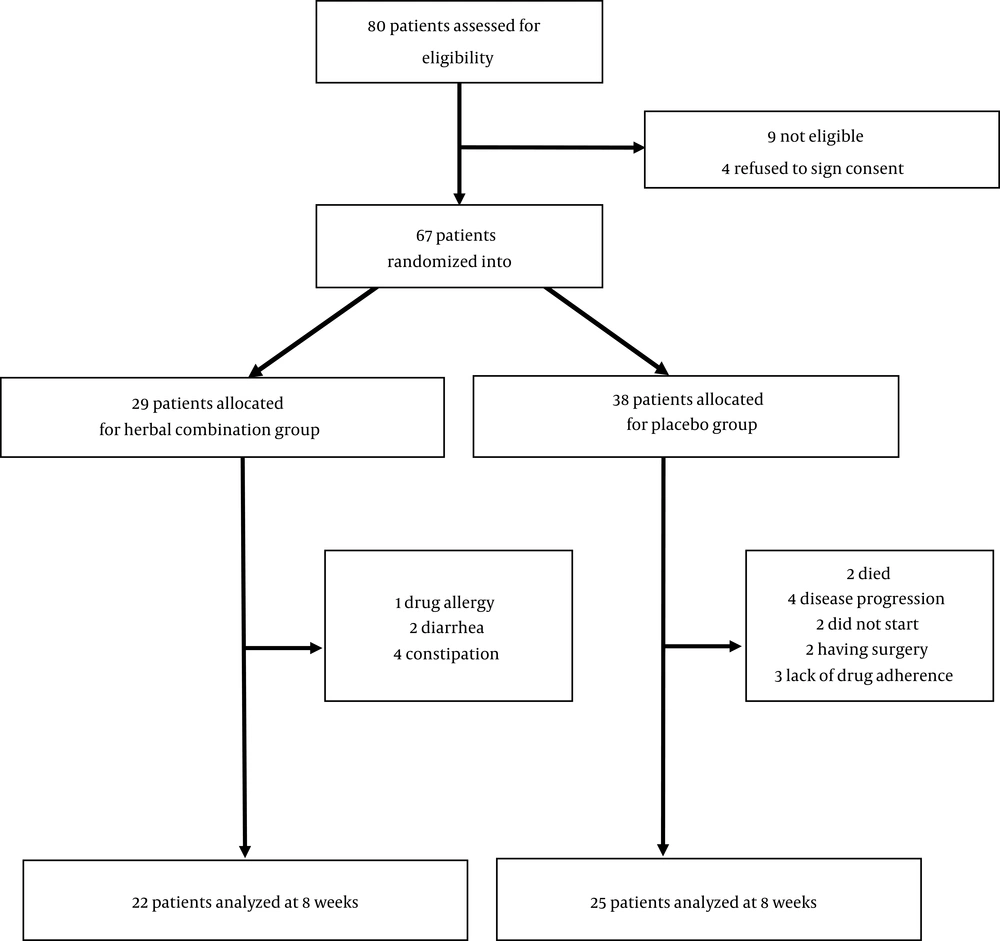
Patients were randomly divided into two groups: (1) Intervention group that received one oral tablet of the herbal combination 3 times daily and (2) Placebo group that received the oral identical placebo tablets 3 times daily. The duration of follow-up was 2 months. The herbal combination tablets were prepared, packaged, and donated by one of the largest herbal companies in Iran and contained a fixed value dose of Trigonella Foenum-graecum (260 mg), Cichorium intybus (260 mg), and Foeniculum vulgare (260 mg). The placebo tablets were also prepared and donated by the same company and they had the same taste, smell, color, and ingredient as intervention group except in their active ingredients (contained starch and chickpea). In this study, the method of sampling was based on blocked randomization by sealed envelopes containing a computer-generated code, and to fulfill the method of distributing tablets in a fully double-blind way, the envelopes were opened by a third person. The process of sampling and randomization to each group as well as data missing during the study were depicted in Figure 1.
3.3. Outcome Measures
3.3.1. Baseline Characteristic
At the baseline, the patients who were eligible for recruiting were selected and the patients’ demographic data, performance status (based on Eastern Cooperative Oncology Group (ECOG), stage of the disease, and detailed medical history were collected through medical information sheet and interview by the main investigator (educated pharmacy student). The height (cm), weight (kg), mid-upper arm circumference (MAC, cm), and laboratory values, including blood count, liver function tests, and inflammatory criteria were recorded at the baseline and after an 8-weeks follow-up.
3.3.2. Anthropometric Indexes
For evaluating the anthropometric indices, triceps skinfold thickness (TSF) and MAC were measured by Harpenden skinfold calipers and stretch resistant tape, retrospectively. Mid Arm Muscle Circumference index (MAMC), which was an indicator of lean body mass, obtained from TSF and MAC criteria according to the equation below. MAMC (cm) = MAC (cm) - [0.314*TSF (mm)]. We also measured grip strength by a digital handgrip dynamometer and all extracted data were recorded for further analyses.
3.3.3. Edmonton Symptom Assessment Scale
The Edmonton Symptom Assessment Scale (ESAS) was designed to assess 10 frequently experienced symptoms by patients, who suffered from cancer within the previous 24 hours including pain, tiredness, nausea, depression, anxiety, drowsiness, anorexia, well-being, shortness of breath, and other symptoms. The ESAS was validated for the assessment of the intensity of symptoms in patients suffering from cancers (18). The severity of each sign was rated from 0 to 10 on a numerical scale, in which 0 and 10 mean the absence of the symptom and the worst possible severity of symptoms, respectively.
3.3.4. Quality of Life
The patient’s quality of life was determined, using the Iranian version of the European organization for research and treatment of cancer (EORTC) of quality of life (QLQ)-C30 version. 3.0 form (European Organization for Research and Treatment of Cancer), which was translated and validated previously by the Iranian researchers (19).
3.4. The Functional Assessment of Anorexia/Cachexia Therapy (FAACT)
The FAACT is a 12-item symptom-specific scale, which was designed to measure patient’s additional symptoms about anorexia-cachexia during the last 7 days. Items were scored from 0 to 4 (0 = not at all, 1 = a little bit, 2 = somewhat, 3 = quite a bit, and 4 = very much). The FAACT has internal consistency and a reliability coefficient (Cronbach’s alpha) of 0.88 for its 12 components (20).
3.5. Adverse Effects and Other Endpoints
During the time of follow-up, every reported adverse effect was noted according to common terminology criteria for adverse events (CTCAE) version 3 (21). Moreover, we defined the primary endpoint of the study as a one-kilogram increase in patient’s weight at the end of the 2-month follow-up and the secondary endpoint as the increase in two-score of the FAACT index and at least 10% enhancement in patients’ quality of life.
3.6. Statistical Analysis
According to the data analyses, the normal distribution of data in both intervention and placebo groups (demographic characteristics of patients) was determined by independent samples t test. Besides, independent samples t test and chi-squared tests were used to analyze the distribution of the parameters between groups. Changes in the questionnaire and anthropometric indices between and within each group were performed by paired-samples t test and independent t test and Mann Whitney u-test. In this study, the Statistical Package for Social Sciences (SPSS) version 20 software was used and P-value less than 0.05 was considered significant.
4. Results
4.1. Baseline Characteristics
Initially, 67 patients were enrolled and randomized into the studies’ arms. According to Figure 1, 7 patients from the herbal combination group and 13 patients from the placebo group were excluded due to various reasons (such as disease progression, drug adverse reaction, and lack of adherence). At the end of the study, 22 patients in the intervention arm and 25 patients in the placebo arm (total patients = 47) succeeded to complete the follow-up duration.
As shown in Table 1, the patients baseline characteristic in both groups of placebo and intervention had no significant differences in term of age (P = 0.49), gender (P = 0.73), baseline weight (P = 0.74), body mass index (BMI) (P = 0.63), type of cancer (P = 0.97), comorbid conditions (P = 0.85). However, the patients enrolled in the herbal combination group had significantly worse ECOG performance status than the placebo group (P = 0.006). In both groups, the majority of patients (more than 50%) were suffering from gastrointestinal cancers. Besides, there was no difference in terms of cachexia grade, FAACT score, and EORTC quality of life between the placebo group and interventional one (P = 0.17, 0.06 and 0.17, respectively).
| Variables | Placebo Group (N = 22) | Herbal Group Combination (N = 25) | P Value |
|---|---|---|---|
| Sex (M:F), No. (%) | 13:9 (59.1, 40.9) | 16:9 (64, 36) | 0.73 |
| Age (y), mean ± SD | 63.1 ± 13.7 | 60.6 ± 11.9 | 0.49 |
| Baseline weight (kg), mean ± SD | 62 ± 8.9 | 61.1 ± 9.5 | 0.74 |
| BMI (kg/m2), mean ± SD | 22.8 ± 3.8 | 22.3 ± 3.1 | 0.63 |
| Type of cancer, No. (%) | 0.97 | ||
| Gastrointestinal | 13 (52) | 16 (53.3) | |
| Breast | 4 (16) | 4 (13.3) | |
| Lung | 4 (16) | 6 (20) | |
| Others | 4 (16) | 4 (13.3) | |
| Past medical history, No. (%) | 0.85 | ||
| None | 8 (32) | 7 (23.3) | |
| Hypertension | 9 (36) | 11 (36.7) | |
| Heart failure | 4 (16) | 4 (13.3) | |
| Benign prostatic hyperplasia | 2 (8) | 5 (16.7) | |
| Others | 2 (8) | 3 (10) | |
| ECOG performance score, No. (%) | 0.006 | ||
| 0 | 2 (8) | 0 (0) | |
| 1 | 10 (40) | 3 (13.6) | |
| 2 | 9 (36) | 10 (45.5) | |
| 3 | 4 (16) | 8 (31.8) | |
| 4 | 0 (0) | 2 (9.1) | |
| Cachexia grade, No. (%) | 0.17 | ||
| 0 | 5 (20) | 2 (9.1) | |
| 1 | 9 (36) | 4 (18.2) | |
| 2 | 2 (8) | 8 (36.4) | |
| 3 | 6 (24) | 3 (13.6) | |
| 4 | 3 (12) | 5 (22.7) | |
| FAACT score, mean ± SD | 23.2 ± 2.8 | 25.4 ± 5.1 | 0.06 |
| EORTC QLQ-C30 score, mean ± SD | 64.4 ±4.9 | 66.9 ±7.7 | 0.17 |
Baseline Characteristics of Eligible Patients
4.2. Weight and Anthropometric Indices
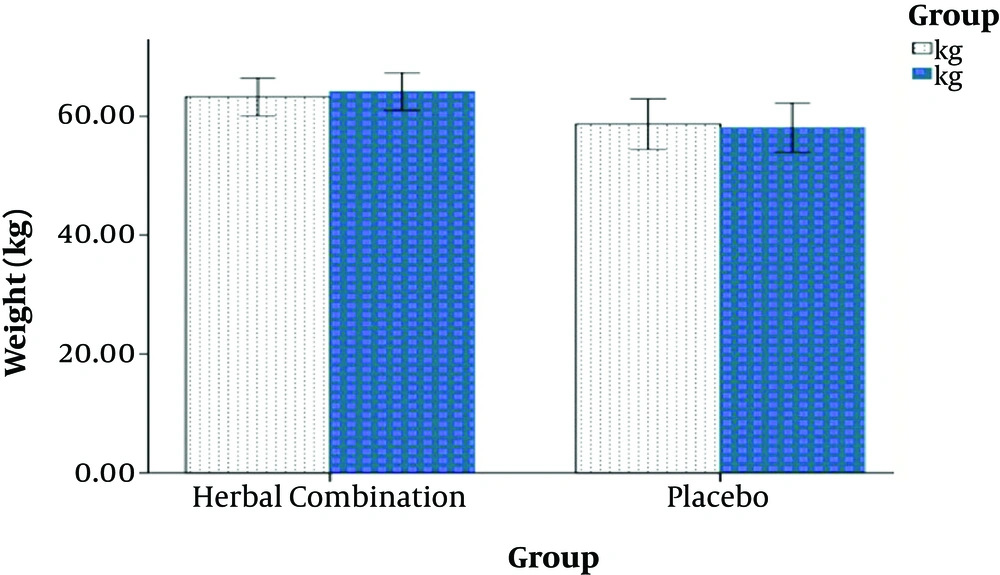
After 8 weeks, the results showed that the changes in body weight and BMI were significantly improved in the intervention group than in the placebo group. Patients in the herbal combination group experienced a mean weight gain of 1.5 kg, but patients in the placebo group had an average weight loss of 0.5 kg. (P < 0.001).
Additionally, there was a significant increase in the other assessed anthropometric indices such as MAMC (P = 0.02), TSF (P = 0.01), and grip strength (P < 0.001) in the intervention group (Table 2 and Figure 2).
| Placebo Group | Herbal Group | Absolute Difference | P Valuea | |
|---|---|---|---|---|
| Weight changes (kg) | -0.5 | 1.5 | 2 | < 0.001 |
| Change in MAMC (cm3) | 0.08 | 0.9 | 0.82 | 0.02 |
| Change in TSF (mm) | 0.3 | 1 | 0.7 | 0.01 |
| Change in grip strength | -0.5 | 2.4 | 2.9 | < 0.001 |
Changes in the Patients’ Weight and Anthropometric Indices After 8 Weeks
4.3. Edmonton Symptom Assessment Scale
The result of the Edmonton scale compromising of 9 essential questions after 8 weeks showed a significant change in 6 indices of pain (P < 0.009), anxiety (P < 0.001), lack of appetite (P < 0.009), malaise (P = 0.02), drowsiness (P = 0.005), and shortness of breath (P = 0.02) in herbal combination group in comparison with baseline (within-group differences). However, in the placebo group, the differences were not statistically significant before and after the intervention (P = 0. 05). Also, according to independent sample t test, the average changes of items such as pain (P = 0.01), anxiety (P = 0.01), drowsiness (P = 0.01), lack of appetite (P = 0.01), malaise (P = 0.04), and shortness of breath (P = 0.02) between herbal combination and placebo groups showed notable differences (P < 0.05) (Table 3).
| ESASS Criteria/Groups | 0 Week, Mean ± SD | 8 Weeks, Mean ± SD | P Value Within- Groupsa | Changes, Mean ± SD | P Value Between Groups |
|---|---|---|---|---|---|
| Pain | 0.01 | ||||
| Herbal | 3.8 ± 0.5 | 2.9 ± 0.5 | 0.009 | -0.8 ± 0.3 | |
| Placebo | 2.2 ± .03 | 2.3 ± .04 | 0.40 | 0.2 ± 0.2 | |
| Fatigue | 0.20 | ||||
| Herbal | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.16 | -0.1 ± 0.1 | |
| Placebo | 0.7 ± 0.3 | 0.9 ± 0.3 | 0.38 | 0.2 ± 0.1 | |
| Nausea | 0.12 | ||||
| Herbal | 3.2 ± 0.5 | 2.6 ± 0.4 | 0.20 | -0.6 ± 0.4 | |
| Placebo | 0.8 ± 0.3 | 1 ± 0.2 | 0.33 | 0.2 ± 0.1 | |
| Depression | 0.33 | ||||
| Herbal | 3.2 ± 0.6 | 2.9 ± 0.5 | 0.18 | -0.3 ± 0.2 | |
| Placebo | 3.4 ± 0.5 | 3.5 ± 0.5 | 0.88 | 0.1 ± 0.3 | |
| Anxiety | 0.01 | ||||
| Herbal | 5.1 ± 0.5 | 4.3 ± 0.4 | 0.001 | -0.8 ± 0.2 | |
| Placebo | 2.9 ± 0.4 | 3.1 ± 0.4 | 0.60 | 0.2 ± 0.3 | |
| Drowsiness | 0.01 | ||||
| Herbal | 4.5 ± 0.4 | 3.6 ± 0.3 | 0.005 | -0.8 ± 0.3 | |
| Placebo | 2.1 ± 0.4 | 2.2 ± 0.3 | 0.72 | 0.08 ± 0.2 | |
| Shortness of breath | 0.02 | ||||
| Herbal | 4.6 ± 0.5 | 3.7 ± 0.3 | 0.02 | -0.9 ± 0.3 | |
| Placebo | 4.2 ± 0.4 | 4.4 ± 0.4 | 0.53 | 0.2 ± 0.2 | |
| Lack of appetite | 0.01 | ||||
| Herbal | 4.9 ± 0.6 | 3.9 ± 0.5 | 0.009 | -1 ± 0.3 | |
| Placebo | 2.8 ± 0.4 | 3 ± 04 | 0.6 | 0.2 ± 0.3 | |
| Malaise | 0.04 | ||||
| Herbal | 4.7 ± 0.6 | 3.9 ± 0.4 | 0.02 | -0.8 ± 0.3 | |
| Placebo | 4.2 ± 0.3 | 4.3 ± 0.4 | 0.88 | 0.1 ± 0.3 |
Edmonton Symptom Assessment Indices in the Intervention and the Placebo Groups (Baseline and After 8 Weeks)
4.4. Quality of Life and FAACT Questionnaire
The results showed that the mean FAACT index (P = 0.05) and the quality of life score (P < 0.001) determined by the Iranian Version of the EORTC QLQ-C30 version 3.0 form were significantly higher in the herbal combination group. However, after 8 weeks, the changes in these items were not significantly different in the placebo group (P > 0.05).
4.5. Adverse Events
There were no grade III or IV toxicities attributed to the herbal combination or placebo groups and only 9 patients had evidence of mild constipation (grade I) in the herbal combination group and 13 in the placebo group. However, the mild adverse events had not halted any drug consumption in each group. We reported 1 patient, who had a drug allergy to the herbal combination and excluded from the study initially.
4.6. Study Endpoints
The primal endpoint in this study was 1-kilogram weight gain after 8 weeks of intervention, which was achieved in 13 patients in the herbal combination group (43%) in comparison with only 2 patients in the placebo group (8%) (P > 0.001). The secondary endpoint was increased in 2-score of the FAACT index and at least 10% enhancement in patients’ quality of life obtained in 20 (68%) patients in a herbal combination group and 8 (21%) patients in the placebo group; the second endpoints were achieved in intervention groups (P > 0.001).
5. Discussion
This study confirmed that the administration of herbal combination contained Fenugreek and Fennel. Chicory has significant beneficial effects on weight gain, improvement in appetite, and quality of life in patients suffering from cancer-induced cachexia and anorexia after 8 weeks’ follow-up without any significant toxicity or complication. The protective mechanism, by which herbal combination led to weight loss attenuation, was not entirely understood. The proposed mechanism arises from the modulation of the inflammatory response by ingredients like Fenugreek and Chicory (16, 17). However, finding the exact effective mechanism of the herbal combination needs to be discovered through the cellular and molecular pathway in animal and clinical studies.
During this preliminary study, the beneficiary effects of the herbal combination were proved. The effects should be checked through serum biomarkers and sort of inflammatory cytokines to assess the meticulous impact of the herbal combination on cellular function.
The administration of anti-inflammatory drugs has been always a matter of concern for attenuating the cascade of inflammation in cancer-induced cachexia and anorexia, although conflicting results have been noted in previous studies (22, 23).
For example, in a pilot study conducted by Lain et al. on patients suffering from head and neck and gastrointestinal cancer-induced cachexia, the administration of celecoxib 200 mg twice daily showed a 1-kilogram weight gain, improvement in BMI and quality of life score, whereas, in the placebo group, the weight loss of 1.3 kg was observed (14). However, the levels of circulating pro-inflammatory cytokine were not statistically different between the treatment and control groups.
In similar studies, in a Japanese clinical trial conducted in 2016, 100 mg anamorelin, an analog of ghrelin, was administrated to the non-small cell lung cancers suffering from cancer-induced cachexia for 12 weeks. The results revealed that anamorelin increased the serum biomarkers such as insulin-like growth factor-1 (IGF-1) IGF-1, IGF binding protein-3 (IGFBP-3), and prealbumin significantly in comparison with the placebo group. However, there was no significant difference in other inflammatory serum markers such as TNF and ILs (23). Again in this study, the significant decline in the level of inflammatory cytokines was not confirmed. Furthermore, according to protective results in weight gain and other criteria, one should conclude that the suppression of proinflammatory cytokine is not the only considered protective mechanism and possibly the other unclear mechanism is in charge and must be revealed.
The other proposed effective mechanism of the herbal combination in improving cancer-induced cachexia is a direct effect of these herbal ingredients on cancer-induced anorexia. For instance, the anti-diabetic and appetizing effects of Fenugreek seeds by enhancing insulin release were proved in different animal (24) and clinical studies (25). In this study, the significant appetite enhancement was seen in our recruited patients and was confirmed, using the Edmonton scale.
The results showed that the 8-week administration of the herbal combination in adjunctive with megestrol (160 mg/day) caused a significant increase in cancer patients’ weight in comparison with placebo combination and megestrol (160 mg/day). The similar promising results have been proposed by numerous agents such as thalidomide (13) and L-carnitine supplementation (15).
For example, in line with our results, Gordon et al. in 2005 in a placebo-controlled clinical trial indicated that 200 mg thalidomide daily has a notable effect on weight and anthropometric indices. Patients in the thalidomide group had gained, on average, 0.37 kg in weight compared with a loss of 2.21 kg (absolute difference 2.59 kg) in the placebo group (13). In a similar trial, L-carnitine (4 gram, daily) has been used for cancer-induced cachexia and significant weight improvement in the interventional group after 6 weeks follow-up was noted (15).
It should be noted that not all examined agents succeeded to show weight enhancement properties. For example, in a randomized clinical trial using cyproheptadine, 8 mg orally 3 times a day in patients with advanced malignancies, cyproheptadine did not significantly abate progressive weight loss in these patients with the advanced fatal disease. Patients who had assigned to cyproheptadine arm lost weight by an average of 4.5 pounds per month compared with 4.9 pounds per month in the placebo arm (26). Although weight gaining properties from cyproheptadine have been reported in the earlier studies, the anti-wasting effects of cyproheptadine were not demonstrated in cachectic patients suffering from cancers.
In another study, Del Fabbro et al. performed a randomized, double-blinded, 28-day trial of melatonin 20 mg versus placebo in patients with advanced lung or gastrointestinal cancers to investigate the effects of melatonin in weight, appetite, and quality of life improvement. After the interim analysis of 48 patients, the study has been halted due to an insignificant difference between comparable variants (27). Furthermore, melatonin failed to show any compelling results in cancer-induced cachexia.
According to our encouraging data, having administrated by the herbal combination in cachectic patients not only led to weight gain but also could improve quality of life, FAACT score, and anthropometric indices during an 8-week follow-up. It reflects this fact that 8 weeks was suitable enough to indicate the positive effects of the herbal combination in the overall health and life condition of patients with cancer.
As mentioned in Gordon et al.’s study (13), thalidomide induced a notable effect on weight and anthropometric indices of patients with cancer while significant improvement in the quality of life after a 4-week follow-up was not shown.
According to the data, it seems that reaching the quality of life improvement needs a longer duration of follow-up in comparison with other indices and the criteria could ameliorate in the studies lasted for more than 8 weeks. For example, in line with our results, Kraft M et al. could demonstrate the notable effects of L-carnitine (4 gram, daily) after a 12-week follow-up while they could not prove any significance after 6-week follow-up. Therefore, one can conclude that changing in some parameters such as quality of life possibly requires more span of times; meanwhile, the shorter follow-up periods seem to be adequate for detecting changes in the appetite and patients’ weight score.
The findings of the study suggested the potential limitations associated with conducting a randomized controlled trial concerning herbal combination for the treatment of cancer-induced cachexia; firstly, the limited number of eligible patients due to the stringent exclusion and inclusion criteria which has been restricted the vast range of patients’ recruitment and, secondly, having a short time of follow-up, limited to 8 weeks due to acceleration in patient’s dropouts and termination in patients’ drug adherence.
Taken all limitations together, as our knowledge serves, this is the only study demonstrating the potential effects of the traditional herbal combination on the management of cancer-induced complications such as cachexia or anorexia. This herbal combination can effectively be used as a safe substitute for proposed chemical medicines such as megestrol with several adverse effects. The concern is rising over the use of megestrol as a standard agent for the management of cancer-induced cachexia, especially for patients who are at risk of thromboembolic events.
Based on the experience from the study, perhaps future trials for assessing the potential effects of agents for the treatment of cancer-induced anorexia/cachexia in patients who have advanced cancer should be conducted in less stringent entry criteria, a larger sample, and longer follow-up. Our suggested herbal combination might also be a more effective therapy if used much earlier in the disease trajectory, and this should be a consideration in the design of any future intervention trial for appetite or cachexia.
5.1. Conclusions
Given the ameliorated results from the herbal combination supplementation contained Fenugreek, Fennel, Chicory in terms of weight gain and appetite improvement as well as physical and quality of life enhancement, it seems that the herbal combination can be used as an adjunctive treatment for patients suffering from cancer-induced cachexia and anorexia. However, further studies with larger sample sizes and longer follow-up periods are warranted.