1. Background
Gastric cancer is the third leading cause of death among cancer types being one of the most malignant diseases. Over 95% of gastric cancer cases are adenocarcinomas. Ovarian cancer is considered the most dangerous cancer in women in particular. Approximately 90% of the malignant tumor of the ovary develops in the surface layer of the ovary, which is called epithelial ovarian cancer (1, 2). Nowadays, several studies are conducted on different plant species to determine their effect on some serious diseases such as cancer threatening human health. Particularly, medicinal plants may possess diverse groups of components such as flavonoids, phenols, tannins, carotenoids, anthocyanins, as well as natural antioxidants that might be cytotoxic to cancer cells and not harmful to normal cells (3). Anthocyanins belong to polyphenolic compounds, which are secondary plant metabolites that act as an antioxidant, anti-inflammatory, cardio-protective, antitumor, and antidiabetic in the human body. It is endogenously available in many foods and plants, which causes them to turn red, purple, or blue (4, 5). Carotenoids belong to the isoprenoids as their structure is made of an isoprene unit. Carotenoids are also secondary plant metabolites that work as antioxidants and have health benefits such as reducing the risk of cancer and eye disease and strengthening the immune system (6, 7). Ocimum basilicum L. (basil) from the Lamiaceae family contains numerous bioactive compounds such as anthocyanin, polyphenols, and other polyterpenes (8). A high concentration of phenolic acid-like, rosmarinic, chicoric, caffeic and caftaric acids, estragole, linalool, and eugenol is found in basil, where it has been used as an herbal medicine for Parkinson’s disease, atherosclerosis, myocardial, and cancer (9, 10). Basil extract has been tested on several cancer cell lines such as human promyelocytic leukemia (HL-60) and neuroblastoma (NB) and many others (11). Impatiens walleriana from the Balsaminaceae family has been found to possess antioxidant or antimicrobial activities. This family has quinines with antimicrobial activity and are used in the treatment of infectious and inflammatory diseases (12).
2. Objectives
The investigation of the anthocyanin and carotenoid types of compounds in O. basilicum and I. walleriana, as well as their cytotoxicity on AGS (human gastric adenocarcinoma) and SKOV-3 (human ovarian carcinoma) cancer cell lines, was the aim of the current study. The cytotoxic effect of I. walleriana on cancer cells has not been reported so far on AGS and SKOV-3 cell lines and O. basilicum was used as a highly common herbal medicine to be compared with I. walleriana.
3. Methods
3.1. Chemicals and Reagents
Ethanol (99.9%), methanol (99.9%), hydrochloride acid (HCl), sodium hydroxide, silver nitrate, distilled water, citric acid, acetone, phenolphthalein, Dulbecco’s modified Eagle’s medium (DMEM), ethylenediaminetetraacetic acid (EDTA), fetal bovine serum (FBS), and trypsin were purchased from Merck company. Annexin V-FITC kit and PI (100 µg/mL) were bought from Medical and Biological laboratories. AGS and SKOV3 cancer cell lines were purchased from Pasteur Institute of Iran.
3.2. Plant Materials
Ocimum basilicum and I. walleriana species were purchased from Bagh Firuzeh, Tehran, Iran in 2017, and approved at Alzahra University herbarium. Leaves of O. basilicum and I. walleriana were used for investigation.
3.3. Total Anthocyanin Amount
Anthocyanin derivatives were extracted directly from O. basilicum and I. walleriana by ethanol/methanol. For this procedure, 0.1 g of each sample was mixed with 10 mL of methanol and HCl. Acidified methanol extraction was performed with 99.9 methanol and 0.1 HCl (v/v). Then, it was stored in a dark place for 24 hours. The supernatants were combined and, then, shed in the test tube, and centrifuged for 11 minutes at 2500 rpm. The absorbance of each acidified methanol extract was measured at 550 nm (13). The following formula was used for calculating anthocyanins concentration:
Anthocyanin content (mg/L) = (A × MW × DF × 1000)/(ɛ × 1)
A: Absorbance (A520 nm- A700 nm) pH 1.0 - (A520 nm- A700 nm) pH 4.5
MW: Molecular weight of cyanidine -3-O- glucoside (449.2 g/mol)
DF: Dilution factor (1 for O. basilicum and 0.5 for I. walleriana)
ɛ: Molar extinction coefficient of cyaniding-3-o-glucoside (26900 L/mmol)
Total anthocyanin amount was calculated as mg per g of fresh weight of the plants.
3.4. Total Carotenoid Amount
For each extract, 0.05 g of the plants were mixed with 5 mL of acetone. The mixture was centrifuged at 2500 rpm for 12 minutes. The absorbance was read at wavelengths of 470, 646, and 664 nm (3). The results were obtained in µmol/g in fresh weight. The following formula was used for calculating chlorophyll a, chlorophyll b, and carotenoid concentration:
Chlorophyll a: 12/25 × A - 2/79 × A
Chlorophyll b: 21/51 × A - 5/01 × A
Carotenoid: (1000 × A - 1/8 × Chl.a - 85/02 × Chl.b)/198
A = Absorbance of the sample
3.5. Cell Culture
AGS and SKOV3 cancer cells were cultured in DMEM containing 10% (5 mL) FBS and 1% (50 Units/ml) Pen-Strep incubated in an incubator with 5% CO2 humidified atmosphere and at 37°C (14).
3.6. Preparation of the Extracts
Water was the most efficient solvent in total antioxidants extraction; therefore, water solvent was selected to determine the cytotoxic effects of extracts on AGS and SKOV3 cancer cell lines (12). The air-dried plant samples (1 g for each) were extracted with 100 mL aqueous solvent for 60 minutes at 70°C temperature. The extracts were centrifuged for 20 minutes at 2000 rpm, the powdered extracts were provided from their supernatants, using the freeze drying process. The extract powders were dissolved in phosphate-buffered saline (PBS) and sterilized with 0.22 mm filters. Then, the solutions were diluted with DMEM to 0.5 mg/mL to 5 mg/mL concentrations (14).
3.7. Cell Viability
3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used for the determination of cell viability of cancer cells. Cells were 0.8 × 104 seeded into 96-well plates and kept for 24 hours in an incubator. After 24 hours, various concentrations of the extracts were added to the cell plates and kept in an incubator for 24, 48, and 72 hours. Subsequently, 10 mL of MTT (5 mg/mL) was added to each well; then, cells were incubated for 4 hours at 37°C. Afterward, 100 mL of dimethyl sulfoxide (DMSO) was added to the wells, and plates were kept for 10 minutes in the incubator. Absorbance was measured at 570 nm wavelength, using a microplate reader. IC50 values were calculated by a dose-dependent curve (14).
3.8. Flow cytometry
AGS and SKOV-3 cells were seeded at a density of 1.6 × 105/well in a 6-well flat-bottomed culture plate and were cultured for 24 hours. Then, each medium was replaced with a serum-free medium and the cells were incubated at 37°C for 48 hours. After incubation, the medium was transferred to a 15-mL centrifuged tube. Then, cells on the culture plate were washed with PBS(-) and were incubated with 1 mL of trypsin solution for 5 minutes at 37°C. After incubation, 0.5 mL of DMEM and 1 mL were added to each plate and mixed well by pipetting. The cell suspension was transferred to the centrifuged tube and was centrifuged for 5 minutes at 300 g. After the supernatant was removed, the cells (1 × 106) were suspended with PBS(-) and were filtrated through 30 µm mesh. The cells were suspended in 100 µL of the binding buffer; then, 10 µL of Annexin V-TIFC and 5 µL of PI were added to the cell suspension and were incubated at room temperature for 15 minutes in the dark to stain the cells. Then, 400 µL of binding buffer was added and the cell sample was measured by flow cytometer (BD FACS Calibur). FITC and PI fluorescence at 525 nm and 620 nm, respectively, were detected and subjected to quadrant analysis of fluorescence intensity, using the Flow JO 7.6.1 software (15, 16).
3.9. Statistical Analysis
The data were expressed as means ± standard deviation (SD). ANOVA test by SPSS version 24 program was used for statistical analysis and P-value < 0.05 showed significant differences.
4. Results
4.1. Total Anthocyanin Amount
The total anthocyanin amount of I. walleriana and O. basilicum extracts were measured as 18.577 ± 0.011 and 12.524 ± 0.031 µmol/g (dry weight), respectively. Anthocyanin amount in I. walleriana was higher than that of O. basilicum.
4.2. Total Carotenoid Amount
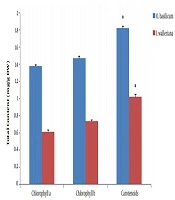
As shown in Figure 1, the total content of chlorophyll a, chlorophyll b, and carotenoid in O. basilicum was higher than I. walleriana.
4.3. MTT Assay Results
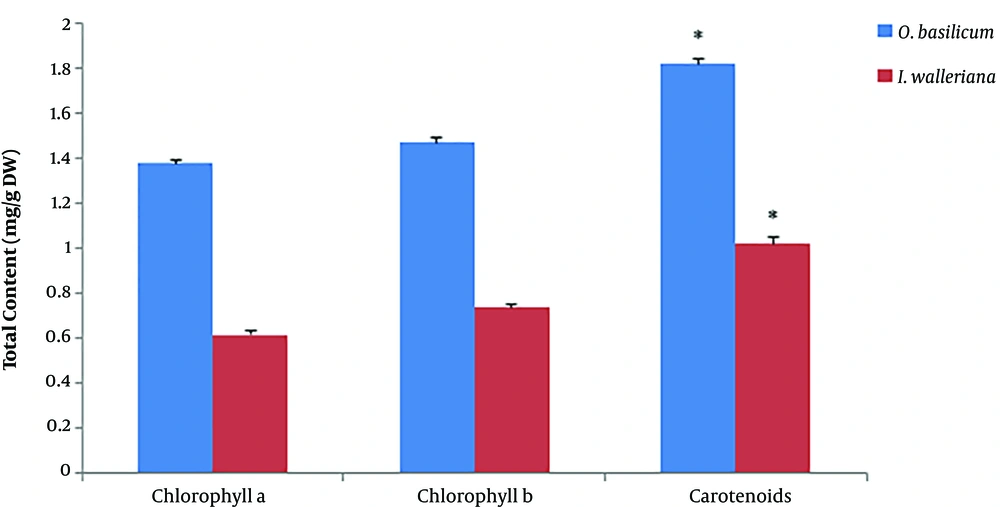
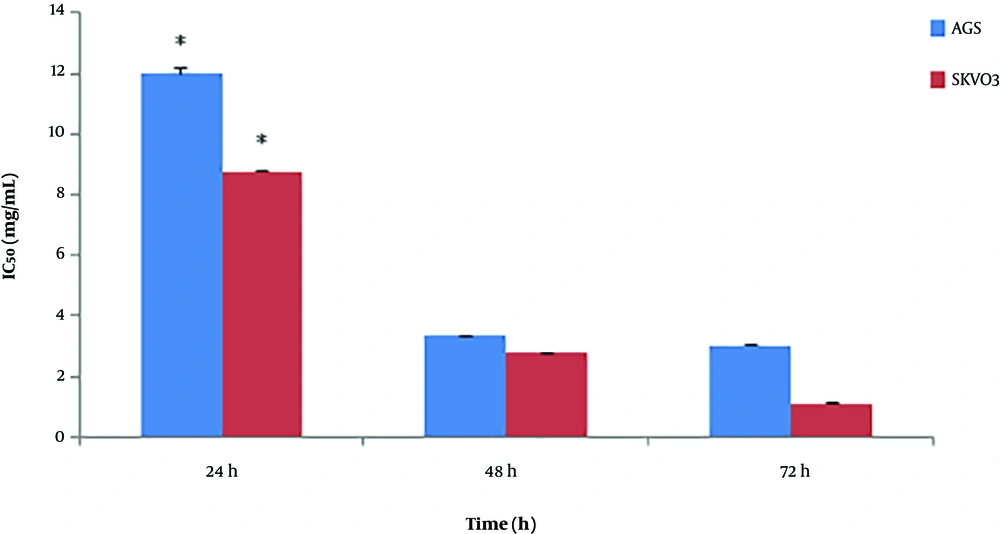
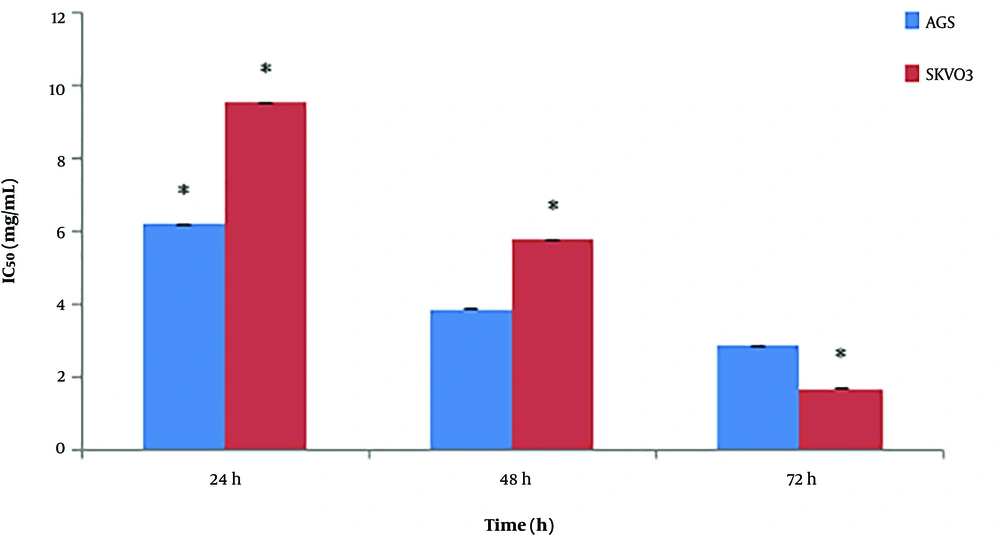
According to the data exhibited in Figure 2, the maximum inhibition of cancer cells growth was on SKOV-3 cell line at 5 mg/mL concentration of O. basilicum within 72 hours with an IC50 value of 0.91 ± 0.11 mg/mL and 91% cell growth inhibition, while the maximum cytotoxic effect of O. basilicum on AGS was at 5 mg/mL concentration within 72 hours with an IC50 value of 3.18 ± 0.02 mg/mL and 78% cell growth inhibition. Impatiens walleriana extract showed the highest cytotoxic effects at 5 mg/mL concentration within 72 hours on AGS with an IC50 value of 2.5 ± 0.21 mg/mL and 89% cell growth inhibition and SKOV-3 with an IC50 value of 1.61 ± 0.07 mg/mL and 87% cell growth inhibition (Figure 3).
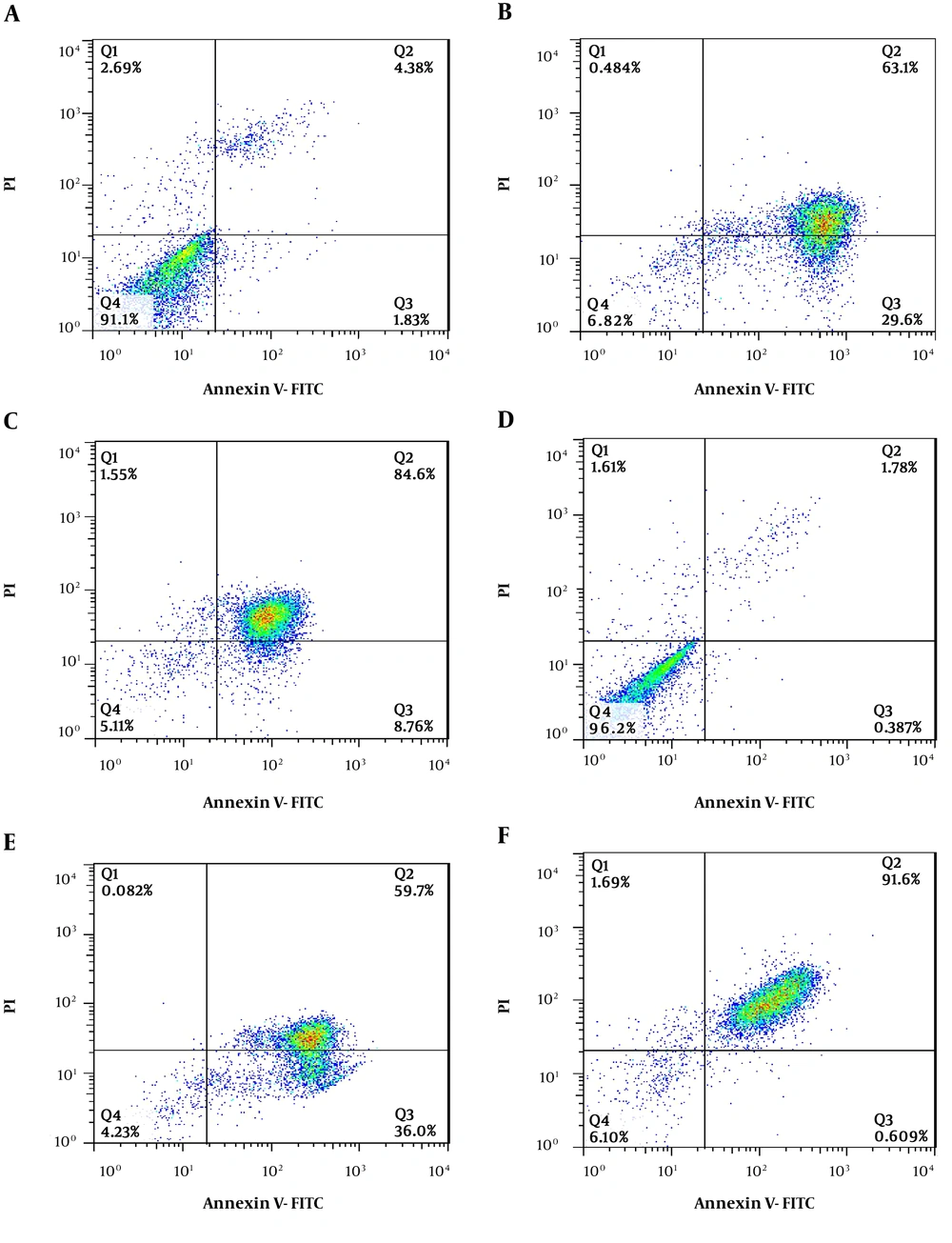
4.4. Flow Cytometry Results
In Figure 4, the lowest left quadrant represents viable cells (Q4), the upper left quadrant represents necrotic cells (Q1), the lowest right quadrant represents early apoptotic cells (Q3), and the upper right quadrant represents late apoptotic cells (Q2). Annexin indicates apoptosis and PI indicates necrosis. Apoptosis is a demonstration by the translocation of phospholipid phosphatidylserine (PS) from the inner to the outer layer of the plasma membrane for phagocyte recognition during the early stage of apoptosis. The extraction of PS to the outer layer is detected by annexin V-FITC for the identification of early apoptosis. PI is a nucleic acid-binding red fluorescent dye, which is impermeable to live cells and early apoptotic cells. It can penetrate to the nucleus and stain the DNA of late apoptotic and necrotic cells when the cytoplasmic membrane integrity is lost. Treatment with O. basilicum and I. walleriana revealed that it induced the exposure of PS on the surface of cells. The results showed a comparison of treated cells with untreated cells. It shows that treatment at IC50 concentration significantly reduced the viable AGS and SKoV3 cells population (Table 1).
Cytotoxic effects of Ocimum basilicum and Impatiens walleriana extracts on AGS and SKOV3 cell lines by flow cytometry analysis: A, AGS cell line control; B, AGS cell line treated with O. basilicum; C, AGS cell line treated with I. walleriana; D, SKOV-3 cell line control; E, SKOV-3 cell line treated with O. basilicum; F, SKOV-3 cell line treated with I. walleriana).
| Compounds | Viable Cell An/PI, % | Early Apoptosis, % | Late Apoptosis, % | Necrosis Cell, % |
|---|---|---|---|---|
| Control AGS | 91.1 ± 0.02 | 1.83 ± 0.02 | 4.38 ± 0.01 | 2.69 ± 0.02 |
| Treatment with I. walleriana extract | 5.11 ± 0.03 | 8.76 ± 0.02 | 84.6 ± 0.02 | 1.55 ± 0.02 |
| Treatment with O. basilicum extract | 6.82 ± 0.02 | 29.6 ± 0.02 | 63.1 ± 0.02 | 0.48 ± 0.02 |
| Control SKOV3 | 96.2 ± 0.02 | 0.38 ± 0.03 | 1.78 ± 0.02 | 1.61 ± 0.02 |
| Treatment with I. walleriana extract | 6.1 ± 0.02 | 0.60 ± 0.02 | 91.6 ± 0.02 | 1.69 ± 0.02 |
| Treatment with O. basilicum extract | 4.23 ± 0.02 | 36.0 ± 0.02 | 59.7 ± 0.02 | 0.08 ± 0.02 |
The Comparison of Results of Flow Cytometry Analysis of the Cytotoxic Effect of Ocimum basilicum and Impatiens walleriana on AGS and SKOV3 Cell Linesa
According to the flow cytometry results, I. walleriana extract showed the highest cytotoxicity on the SKOV-3 cell line and induced cell death with about 93.29%; however; on the other hand, the most effective extract on the AGS cell line was O. basilicum that induced cell death with about 63.58%.
5. Discussion
Cytotoxic effect of Ocimum basilicum and Impatiens walleriana extracts at various concentrations were investigated on AGS and SKOV3 cell lines. The extracts showed cytotoxic effects in a time- and dose-dependent manner. According to the results presented in Figures 2 and 3, the lowest IC50 value with an amount of 2.5 ± 0.21 mg/mL indicates that the most cytotoxic extract on the AGS cancer cell line belonged to I. walleriana extract. On the other hand, the lowest IC50 value of O. basilicum was about 0.91 ± 0. 11 mg/mL on the SKOV3 cancer cell line.
The cytotoxic effect of I. walleriana extract on cancer cells has not been reported up to date, whereas several studies have revealed anti-cancer properties of O. basilicum.
Zarlaha et al. in their study showed that O. basilicum methanol extract had cytotoxic properties on MCF-7 cancer cells. Zarlaha et al. (17) examined the anti-cancer effect of O. basilicum ethanol extract on HeLa and SKOV3 cancer cells and it was concluded that rosmarinic acid, linalool, and caffeic acid in basil had a cytotoxic effect on ovarian cancer cells. They also found that caffeic acid had the most anti-cancer and inhibitory properties on DNA synthesis. Mahmoud GI’s study (11) showed that basil essential oils may be potentially used as good sources of antioxidants and anti-cancer. Basil essential oil was more effective on the HL-60 cell line than the NB4 cell line.
The investigations of Sameie et al. (18) and Juliani and Simon (19) on antioxidant activities in various basils species pointed out the fact that the flavonoid derivative compounds and antioxidant activities in purple basil are more abundant than green ones, which may lead to the conclusion that the antioxidant activities were probably directly related to the number of polyphenols in basil.
The anti-cancer potential of polyphenolic compounds in plants is well documented and natural compounds are a suitable option for the synthesis of safe anti-cancer drugs without any side effects and harming normal cells; however, chemical anti-cancer drugs have various side effects that pose risks for normal cells. Polyphenols showed their anti-cancer effects through manipulation of multiple biological mechanisms involved in cancer initiation and progression such as p53 signaling pathways; therefore, herbal extracts offer comprehensive therapeutic effects more than single drugs (20).
Based on the flow cytometry analysis, both O. basilicum and I. walleriana were noticed to induce apoptosis in the cancer cell lines tested. Impatiens walleriana with a higher content of total anthocyanins was found to induce apoptosis more significantly on SKOV-3 cell lines while O. basilicum showed the highest cytotoxicity on the AGS cancer cell line.
5.1. Conclusions
The extracts of O. basilicum and I. walleriana are rich sources of antioxidants that also show anti-cancer effects. The cytotoxic effect of the extracts on AGS and SKOV3 cancer cell lines can be mainly due to the presence of anthocyanin and flavonoid derivatives. Impatiens walleriana was found to contain a higher amount of anthocyanins compared to O. basilicum. While O. basilicum had the most cytotoxic effects on the AGS cancer cell line, I. walleriana was more efficient on the SKOV-3 cancer cell line. Further studies for identification and isolation of bioactive compounds in these plants and investigation of compounds anti-cancer effect on different cell lines are required.