1. Context
Breast cancer is among the main causes of human distress and mortality that includes 30% of all new cancers diagnosed in females; thus, it is a major public health issue. Breast cancer stands out as the most common cancer worldwide and first cause of deaths related to cancer in females (1).
Breast cancer patients are consistently at risk of skeletal complications as bone is the most common site of metastatic involvement. As a result, osseous-related events (SREs) such as pathologic fractures, hypercalcemia, and pain of the bone, or the need for palliative radiation may transpire as composite outcomes (2). Accordingly, the emergence of bone health maintenance in breast cancer patients is known as an indispensable aspect in survival and morbidity improvement in these patients. Among the varied therapeutic approaches for protecting bone health in breast cancer patients, bisphosphonates play a substantial role in the prevention/delaying of cancer therapy-induced bone loss and SREs in bone-metastatic breast cancer. Moreover, bisphosphonates have revealed an anti-tumor effect that suits them for breast cancer prevention and adjuvant therapy in the early stages of the disease (3, 4).
Bisphosphonates are pyrophosphate analogs that avidly bind to hydroxyapatite crystals of the bone and impede the osteoclastic bone resorption through decreasing osteoclast progenitor development and recruitment and inducing osteoclast apoptosis. Bisphosphonates are categorized into two types: (1) Non-Nitrogen-containing bisphosphonates (such as etidronate and clodronate); (2) nitrogen-containing or amino bisphosphonates (such as zoledronate, pamidronate, risedronate, and alendronate). Zoledronic acid (ZOL), as the most potent bisphosphonate, has illustrated inhibitory effects on migration, invasion, and metastasis of breast cancer cells. Therefore, not only bisphosphonates are suitable armamentarium for the preservation of bone health and controlling SREs in an adjuvant therapy setting, but also they perform an inevitable role in the prevention of bone metastases in breast cancer patients (3, 4).
Recent studies indicate that bisphosphonates are bone-modifying drugs used in patients involved in breast cancer and bone metastases to minimize the incidence of SREs (5, 6). Drug structure of bisphosphonates is similar to pyrophosphates and have a high affinity for hydroxyapatite, primarily which decrease the activity of osteoclasts. Previous reports have shown that bisphosphonates prescribed as adjuvant treatment for postmenopausal women with initial stages of breast cancer may prevent recurrences, postpone the onset of bone metastases, and improve breast cancer-specific and overall survival (7, 8). Although, not all studies indicate an association between adjuvant bone-modifying agent and improved results, however, a meta-analysis declared that the advantages of adjuvant bisphosphonate therapy in breast cancer were limited to a reduction in bone metastases with no effect on mortality of cancer. On the other hand, postmenopausal stage females who received bisphosphonates had lower rates for recurrency and improved breast cancer-specific survival (9).
Despite the advantageous benefits of bisphosphonate and some drugs, they may give rise to a serious condition entitled: Medication-related osteonecrosis of the jaw (MRONJ), which is described as a bone which is necrosed in maxillofacial region persisting for at least 8 weeks, in patients with previous or current treatment of BPs or antiangiogenic drugs (denosumab), and without any history of radiotherapy of the head and neck region. This disease is commonly initiated by a dentoalveolar trauma (dental extraction as the most frequent trigger) or it may spontaneously emerge in rare cases. The fact that MRONJ has most commonly been associated with bisphosphonates administration puts breast cancer patients in constant danger of this disease. MRONJ with a 3% to 5.3% prevalence among breast cancer patients has become a relevant and serious condition (10, 11). van Hellemond et al. (12) reported that no association was observed between reduction of bone mineral density (BMD) and distant recurrence-free survival. Neither did they observe an impact of bisphosphonates on distant recurrence-free survival.
Finally, doses of bisphosphonate used for adjuvant therapy are lower than those used for the treatment of bone metastases, but they are greater than the doses used for treating osteoporosis (5). In this regard because of serious adverse events, such as osteonecrosis of the jaw, the route of administration is important (13). So far, few studies have investigated the effects of bisphosphonates administration on breast cancer patients. In this review of the literature, we aimed at identifying, describing, and summarizing high quality, updated evidence on bisphosphonates administration, biomarkers representative of the efficacy of BP therapy, and MRONJ affection in breast cancer patients to provide comprehensive information on considerations in bisphosphonates prescription and emphasizing the pivotal necessitation of interdisciplinary communication between oncologists, GPs, and dentists for MRONJ prevention in these patients.
2. Methods
2.1. Publication Search
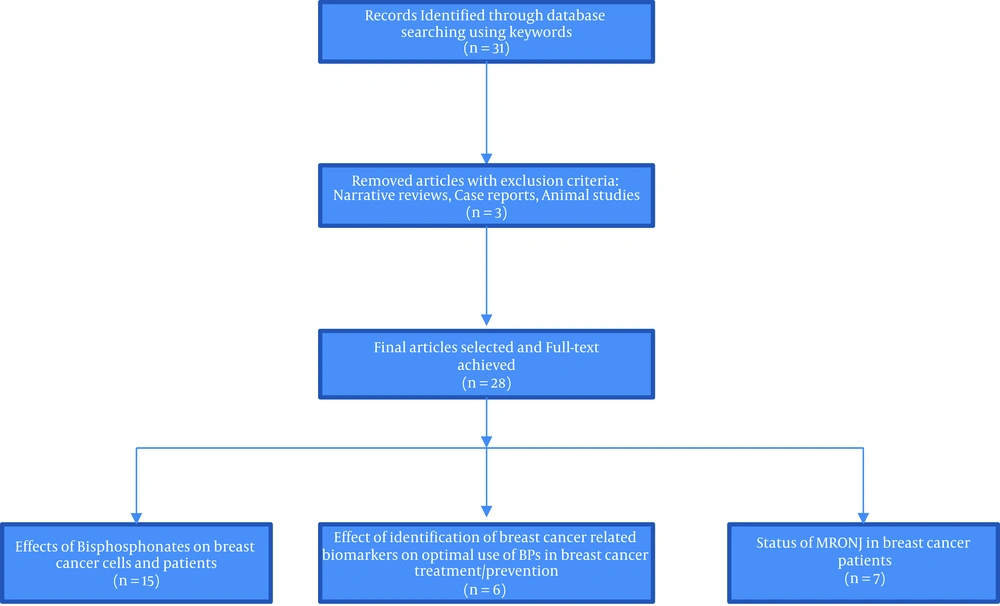
We conducted an overview of the English-language literature involved bisphosphonates administration, cancer biomarkers for bisphosphonate therapy, and bisphosphonate-related osteonecrosis of the jaw (BRONJ) in breast cancer. The electronic databases in PubMed, MEDLINE, and EMBASE were searched in October 2020 for reporting the outcomes of bisphosphonate therapy in breast cancer patients. Reference lists of published papers were, then, hand-searched in an attempt to identify further studies. The following keywords were used: ‘diphosphonates’ (according to MeSH), ‘osteonecrosis’, ‘breast cancer’, and ‘biomarker’. The search terms were, then, entered into Google Scholar to ensure that articles were not missed. The inclusion criteria of the article were “meta-analysis, clinical trials, and randomized controlled trial” published in English from October 2015 to October 2020. Papers were excluded if they were case reports, animal studies, not written in English, lacked documentation, narrative reviews, studies which had no clinical outcomes data, systematic reviews without meta-analysis, and technique articles without outcomes. We, then, obtained full text for those studies that met the inclusion criteria.
2.2. Data Extraction and Classification
Twenty-eight high quality and relevant articles were classified into 3 categories based on their content for answering the following questions (Figure 1):
1) What are the effects of bisphosphonates on breast cancer cells and patients?
Fifteen articles answered this question.
2) How does the identification of breast-cancer-related biomarkers affect the optimal use of BPs in breast cancer treatment/prevention?
Six articles answered this question.
3) How is the status of MRONJ in breast cancer patients?
Seven articles answered this question.
Differences between selected studies regarding their various types of studies, follow-up periods, stage of breast cancer, type of BPs administrated, etc. prevented a valid mathematical combination of the collected data.
3. Results
3.1. Bisphosphonates Administration in Breast Cancer Patients
Features of the related studies are summarized in Table 1.
| Author | Type of the Study | Studied Population | Purpose of the Study | Type of BP Administration | Evaluated Parameters | Study Design/Group Allocation | The Average Follow-up Period | Results | Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Li et al. (14) | A systematic review and meta-analysis | Thirty-four articles were included in this study (4,508,261 participants; 403,196 cases). | To evaluate the effect of bisphosphonates on overall cancers. | Non-nitrogen-containing bisphosphonates | Risk of all-cause cancer. | 4,508,261 participants; 403,196 cases. | - | Bisphosphonates are significantly associated with a risk reduction of colorectal, breast, and endometrial cancer, especially nitrogen-containing bisphosphonates. | Non-nitrogen-containing bisphosphonates might increase the risk of liver and pancreas cancer. Large prospective cohort studies are needed to find the causal association between bisphosphonates and the risk of cancers. |
| Suarez-Almazor et al. (5) | RCT | 37,724 women aged ≥ 66 years with breast cancer | To investigate the association between therapy with bone-modifying agents (BMAs) and survival in older women with early breast cancer. | IV zoledronic acid and ibandronate, oral bisphosphonates and denosumab | Overall survival, breast cancer-specific survival | 1) 6 months with drug claims for an oral bisphosphonate; 2) 2 claims for intravenous ibandronate; or 3) at least 1 claim for either denosumab or zoledronic acid. | The Median follow-up was 64 months | The receipt of a bisphosphonate was associated with improved overall survival and breast cancer-specific survival after multivariable adjustment. | Bisphosphonates at the doses recommended for osteoporosis are associated with improved survival in older postmenopausal women with early breast cancer |
| Yang and Yu (15) | A systematic review and meta-analysis of dosing frequency | 4 articles with available data from 4 randomized clinical trials | To contrast the efficacy and safety of treatment strategies. | Intravenous and oral | Skeletal-related events, renal dysfunction, and osteonecrosis of the jaw | Standard (every 4 weeks) and de-escalation (every 12 weeks) treatment of bisphosphates. | - | There existed no significant difference in on-study skeletal-related events, renal dysfunction, and osteonecrosis of the jaw. | De-escalation treatment with bisphosphates may be superior to standard treatment in terms of efficacy, safety, and economic costs. |
| van Hellemond et al. (12) | Sub‑study of the DATA trial | 1860 eligible patients with a BMD measurement within 3 years after randomization (landmark) without any DRFS events | Evaluation of the effect of bisphosphonates on distant recurrence-free survival. | Anastrozole | Bone mineral density- osteoporosis | Patients used anastrozole for 6 or 3 years according to randomization | Median follow-up of 5.0 years | The BMD was normal in 436 (38.2%) and showed osteopenia in 565 (49.5%) and osteoporosis in 141 (12.3%) patients. | No association was observed between a reduced bone mineral density and DRFS. |
| Eguia et al. (16) | Review | A narrative bibliographic review on drugs related to the development of osteonecrosis of the jaw | To update the list of medications associated with osteonecrosis of the jaw | Different methods | Efficacy of drugs on preventing osteonecrosis of the jaw | - | - | The latest drugs identified as potential facilitators of this pathology include several anti-VEGF based antiangiogenic drugs and anti-TKI and different types of immunomodulators | To prevent new cases of MRONJ, it is essential for all oral healthcare professionals to be fully up-to-date |
| Drieling et al. (17) | RCT | Postmenopausal women diagnosed with breast cancer (n = 887) | Comparison of the short- and long- term effect of oral BP administration on fracture risk | Oral BPs | Fracture risk | G1: 2 - 3 years (31%); G2: 4 - 7 years (36%); G3: 8+ years (33%) | 3.7 years | ≥ 8 years of BP use was associated with a significantly higher risk of fracture while 4 - 7 years of BP use revealed no significant effect on fracture risk. | Longer duration of BP therapy leads to higher fracture risk due to loss of effectiveness over time in postmenopausal women with breast cancer. |
| Hortobagyi et al. (18) | RCT | 416 women with bone metastases from breast cancer who previously received 9 or more doses of zoledronic acid and/or pamidronate during the first 10 to 15 months of therapy. | To examine whether ZOL every 12 weeks was non-inferior to ZOL every 4 weeks in patients with bone metastatic breast cancer | 4 mg zoledronic acid, IV | 1) Skeletal-related events (SREs); 2) safety assessment/adverse events (AEs); 3) skeletal morbidity rate (SMR) | G1: ZOL every 4 weeks n = 200; G2: placebo n = 13; G3: ZOL every 12 weeks n = 203 | 1 year | Neither the time to first SRE nor the SRE-free survival showed a statistically significant difference. SMR was not significantly different between groups. The safety profile of the groups was comparable with 47.5% versus 42.6% of the patients experiencing grade 3 or 4 AEs in every 4 weeks and every 12 weeks groups, respectively. | ZOL regimen of every 12 weeks revealed to be non-inferior to an every 4 weeks regimen for efficacy with a similar safety profile. The effect of long-term ZOL treatment on bone saturation and bone retention rate with its clinical outcome should be further investigated. |
| Rennert et al. (19) | Nested case-control study | 3 731 postmenopausal women with breast cancer who did not use BPs before diagnosis. | The association of use of oral BPs after breast cancer diagnosis on overall and breast cancer survival. | Oral BPs Second-generation BPs: alendronate/ risedronate | 1) Overall survival; 2) breast cancer-specific survival; 3) survival rate based on hormone-receptor of the breast cancer | Non-survivals n = 799 survivals n = 2932 | 70 months | BP administration was revealed to be significantly more common among the survivors rather than in those who died with similar tumor grade/stage. Similar but statistically underpowered survival rate improvements were revealed when the groups were compared based on hormone-receptors of their breast cancer. | Oral bisphosphonates in previously unexposed women diagnosed with breast cancer for at least 18 months improve the odds of surviving breast cancer and overall survival rate. |
| Kroep et al. (20) | Meta-analysis | Pool of individual patient data from 4 prospective randomized clinical trials reporting the effect of the addition of ZOL on the pathological response after neoadjuvant therapy | Assessment of the effect of BPs addition to adjuvant therapy on survival improvement in postmenopausal breast cancer patients | 4mg zoledronic acid, IV | 1) pathological complete response in the breast (pCRb); 2) pathological complete response in the breast and lymph nodes (pCR) | A total of 735 and 552 patients were included for the pCRb and pCR status assessment after neoadjuvant chemotherapy with ZOL | - | ZOL addition to neoadjuvant CT did not increase pCRb or pCR rates. However, in postmenopausal patients, the addition of ZOL resulted in a significant, near doubling of the pCRb rate and a non-significant benefit of the pCR rate. | The addition of ZOL to systemic therapy illustrates survival improvement in postmenopausal women with low levels of reproductive hormone. |
| Liu et al. (21) | Meta-analysis | 7 clinical trials comparing ZOL vs pamidronate and ZOL /ibandronate vs placebo | Efficacy of BPs in treating or reducing the risk of SREs in breast cancer. | - | Risk of new SREs development | - | - | A statistically significant 38% reduction in the risk of developing new SREs with bisphosphonates was revealed. P = 0.000 | BPs are the central therapy for bone metastases with proved efficacy in both treating and reducing the risk of SREs in breast, lung, and prostate cancer. |
| Gralow et al. (22) | RCT | 5400 stage I-III breast cancer patients over 4 years | Comparing the efficacy of 3 bisphosphonates in early-stage breast cancer. | 1) ZOL; 2) Clodro;nate 3) Ibandronate | 1) Disease-free survival; (DFS) 2) overall survival (OS); 3) toxicity (including pain, osteonecrosis of the jaw, GI toxicity, and fracture rates) | 1) ZOL group (n = 2000); 2) clodronate group (n = 2000); 3) ibandronate (n = 1400) | 5 years | 5-year DFS and OS showed no significant difference between the groups of the study. Grade 3/4 toxicity was 8.8% (zoledronic acid), 8.3% (clodronate), and 10.5% (ibandronate). Osteonecrosis of the jaw (ONJ) was the highest for zoledronic acid (1.26%), compared to clodronate (0.36%) and ibandronate (0.77%). | No evidence of a significant difference was found regarding the efficacy of ZOL, clodronate, and ibandronate. ZOL should be prescribed cautiously due to its higher risk of ONJ development. |
| Li et al. (14) | Systematic review | Thirty-four articles | Analyze possible association between the use of bisphosphonates and the risk of overall cancers | Alendronate, Risedronate, Etidronate, Ibandronate, Clodronate, Pamidronate, Zpledronate | Use of bisphosphonates and various types of cancers based on differed types and duration of bisphosphonates | - | - | Bisphosphonates significantly decreased the risk of colorectal cancer, breast cancer, and endometrial cancer. Non-nitrogen-containing bisphosphonates tended to increase the risk of liver and pancreas cancer | The use of bisphosphonates is associated with a decreased risk of colorectal, breast, and, endometrial cancer. Nitrogen-containing bisphosphonates appear to have more anti-tumor effects. The use of bisphosphonates for at least 1 year has a greater protective effect on breast cancer than their use for less than 1 year. |
| van Hellemond et al. (12) | Sub-study of the DATA trial | Postmenopausal breast cancer patients | Assess the relationship between a reduced bone mineral density (BMD) and distance-free survival (DRFS), and evaluated the effect of bisphosphonates on DRFS | Alendronate, Risedronate, Clodronate, Ibadronate, Pamidronate, Zoledronate | To evaluate the effect of bisphosphonates on late DRFS | Optimal duration of adjuvant anastrozole G1: 6 years, G2: 3 years | 5 years | There was no association between a reduced BMD and a lower breast cancer recurrence risk | No association between BMD and late DRFS in this pre-planned DATA sub-study. There was no relationship between bisphosphonate use for a decreased BMD and late DRFS. |
| Yang and Yu (15) | Meta-analysis and systematic review | 4 RCTs | To contrast the efficacy and safety of these two treatment strategies | Zoledronate, Pamidronate | Skeletal-related events, renal dysfunction, osteonecrosis of the jaw | Administration of Bisphosphonates G1: 12 weeks, G2: 4 weeks | Not mentioned | De-escalation of bisphosphonates was non-inferior to standard treatment (every 4 weeks) in terms of reducing the study on skeletal-related events in breast cancer patients | Bisphosphonates every 12 weeks was non-inferior to standard treatment and was more affordable for the breast cancer patient with bone metastasis. |
| Suarez-Almazor et al. (5) | Retrospective cohort study | 33 724 women in older postmenopausal age | To investigate the association between therapy with bone-modifying agents (BMAs) and survival in older women with early breast cancer. | Zoledronic acid | Date of diagnosis until death (overall survival)-breast cancer-specific survival (BCSS) in the database. | A Zoledronic acid and Ibandronate (IV bisphosphonates) B, Alendronate, Ibandronate, Risedronate) D, Denosumab | 64 months | Receipt of bisphosphonates was significantly associated with improved overall survival for patients, who had stage II disease | Early use of low-dose bisphosphonates, including oral agents, may be as beneficial as the more intense regimens recommended for adjuvant therapy. |
Drieling et al. (17) implemented a study on postmenopausal women with breast cancer to examine the association of long-term (more than 8 years), intermediate (4 - 7 years), and short-term (2 - 3 years) oral bisphosphonates use with fracture risk among these patients. The authors considered any self-reported clinical fracture as the outcome of interest during all years of follow-up. Among the 142 clinical fracture reports, patients with long-term consumption of oral bisphosphonates showed the highest unadjusted fracture rate (7.66%), whereas the short-term use of bisphosphonates led to the least fracture rate (4.74%). The authors concluded that the higher fracture risk during long-term administration of oral bisphosphonates may be representative of a decrease in the effectiveness of BPs over time, lower BP adherence in long-term use, or residual confounding factors that should be investigated in future studies. Moreover, they emphasized the importance of safety recommendations for regular reevaluation of long-term BP users for the appropriateness of continuing the BP therapy for breast cancer patients.
Investigating the efficacy of BPs as a major therapy for bone metastases, Liu et al. (21) implemented a meta-analysis on 7 clinical trials appraising the effect of BPs on the risk of SREs. Based on the ground of their study, BP therapy in bone metastatic breast cancer (BMBC) patients leads to a 38% decline in new SREs development.
In another study by Hortobagyi et al. (18), the effect of continued treatment of ZOL dosing was assessed in BMBC patients; 416 patients were randomized to receive 4mg of intravenous ZOL every 4 or 12 weeks. Their study revealed no significant difference regarding factors such as SREs, the time to the first SRE, and skeletal morbidity rate (SMR), which illustrates that ZOL dose reduction to one-third will not cause any significant inferiority in the maintenance of bone health in BMBC patients. In terms of treatment-emergent adverse events (AEs), indicative of the safety profile of ZOL dosing, every 12 weeks group showed less stage 3 or 4 AEs, lower increase in blood creatinine level, lower mortality rate, and no cases of osteonecrosis of the jaw compared with the 4 weeks group. Finally, using a noninferiority margin of 10%, the authors suggested the low-dose ZOL regimen in BMBC patients.
Rennert et al. (19) performed a nested case-control study with the main outcome of all-cause mortality in postmenopausal women with newly diagnosed breast cancer. A large cohort of 3731 breast cancer patients was followed up and assessed for an average time of 70 months in terms of overall and breast cancer-related deaths, hormone receptors such as estrogen receptor [ER], progesterone receptor [PR] and Her2neu condition. Their study demonstrated a significantly decreased mortality in patients with more than 18 months of BP consumption compared with patients with no or less than 18 months with BP intake (P = 0.01). Surprisingly, this point remains significant even after tumor stage and grade adjustment, restricting to deaths only due to breast cancer, and exclusion of women with metastatic diseases. A similar beneficial effect, but statistically not significant, was revealed in ER-positive breast cancers, ER-negative tumors, triple-negative tumors, and HER2neu-positive tumors.
Kroep et al. (20) investigated the effects of ZOL application in neoadjuvant chemotherapy for stage II/III breast cancer patients in terms of pathological response improvements. Data were pooled and analyzed from 4 clinical trials based on pathological complete response in the breast (pCRb)- status, defined as the loss of invasive tumor cells in the breast, and complete response of pathology in the breast and lymph nodes (pCR)-status. According to the results of their study, the addition of ZOL to neoadjuvant chemotherapy failed to depict significant improvement in pCRb or pCR status. ZOL could result in a non-significant improvement with regards to pCRb and pCR in postmenopausal breast cancer patients, but not in pre/perimenopausal patients.
Gralow et al. (22) compared the 3-year administration of ZOL, clodronate, and ibandronate as adjuvant therapy in phase I-III breast cancer patients to assess the disease-free survival (DFS), overall survival (OS), and toxicity of each of the BPs. The results of their study neither revealed a significant difference in DFS nor OS. In terms of toxicity, the oral agents caused higher rates of GI toxicity compared with ZOL. However, despite the low toxicity grade of all the arms of the study, ZOL showed the highest risk for developing osteonecrosis of the jaw among them.
Li et al. (14), with this belief that it is not obvious whether bisphosphonate is related to the risk of cancers, conducted a meta-analysis efforted at evaluating the effect of bisphosphonates on overall cancers. Their results show that bisphosphonates significantly decreased the risk of colorectal cancer, breast cancer, and cancers of endometrium, but no significant association was observed in cancers with all-causes. Besides, bisphosphonates containing nitrogen only had protective effects on breast cancer and also endometrial cancer. bisphosphonates without nitrogen increased the risk of liver cancer and pancreas cancer. They concluded that bisphosphonates are significantly related to the risk reduction of breast and endometrial cancer. It needs to be declared that that bisphosphonates without nitrogen might increase the risk of liver and pancreas cancer.
Li et al. (14) in 2020 published a systematic review and concluded that especially nitrogen-containing bisphosphonates significantly decreased the risk of colorectal, breast and endometrial cancer. They also found that bisphosphonates without nitrogen might increase the risk of liver and pancreas cancer.
van Hellemond et al. (12) in 2018 studied the relevance between a reduced BMD and distant recurrence-free survival (DRFS) of breast cancer patients and evaluated the effect of bisphosphonates on DRFS. After 5 years of follow-up, Osteopenia and psteoporosis were not related to DRFS. They concluded that no relation was observed between a reduced Bone marrow density and DRFS and there was no impact of bisphosphonates on DRFS of breast cancer patients.
Yang and Yu (15) in 2020 in a systematic review compared the efficacy between standard method (every 4 weeks treatment) and decreased method (every 12 weeks treatment) protocol of bisphosphonates in the management of bone metastasis in breast cancer patients and found no significant difference on SREs, renal dysfunction, and osteonecrosis of the jaw, but patients who received IV bisphosphonates before enrollment experienced less SREs and a significant difference was observed between groups. They concluded that decreased method with bisphosphonates may be better than standard treatment in aspects of efficacy, safety, and economic values. But, it is better that all the patients could be treated with bisphosphonates every 4 weeks for several months before decreased method.
Suarez-Almazor et al. (5) in a retrospective cohort study in 2020 investigated the relation between treatment with bone-modifying agents (BMAs) and survival in older females with early breast cancer; 21% of patients received minimum of 6 months of BMAs within the first 2 years of breast cancer diagnosis, including bisphosphonates in 80.7% of patients, denosumab in 15.2%, and both in 4.1%. They concluded that bisphosphonates at osteoporosis treatment dosage are related to increased survival in older postmenopausal females with early breast cancer.
3.2. Assessment of Identified Breast Cancer Biomarkers that are in Correlation with BPs Treatment Outcomes
Features of the related studies are summarized in Table 2.
| Authors | Biomarker | Function in Breast Cancer | Response to BPs |
|---|---|---|---|
| Sandholm et al. (23) | CD73 | High CD73 expression in breast cancer is representative of cancer cell invasion-promoting properties. CD73 is associated with poor prognosis in triple-negative breast cancer (TNBC). | Low-CD73 tumors could benefit more from BP therapy, compared with high-CD73 tumors. CD73 expression may affect treatment responses to BPs in TNBC. |
| Westbrook et al. (24) | DOCK4 | Identifies bone recurrence. Predicts response to ZOL adjuvant therapy. High DOCK4 is significantly associated with aggressive disease and metastasis. | ZOL treatment counteracts the higher risk for bone recurrence from high DOCK4-expression tumors. High DOCK4 expression can be abolished by ZOL. Therefore, high DOCK4 is a predictive biomarker for the prevention of bone metastases by ZOL. ZOL appears to reduce the risk of bone metastases of breast cancer in both high and low DOCK4 tumors. |
| Coleman et al. (25) | MAF | A prognostic biomarker of bone metastasis prevention or treatment in breast cancer. Predicts the likelihood of benefit from adjuvant therapy with ZOL. | About 80% of MAF-negative tumors tend to benefit from ZOL adjuvant. MAF-positive tumors are associated with adverse disease outcomes and, therefore, BP adjuvant therapies in non-postmenopausal, MAF+ patients are recommended to be avoided. |
| Sandholm et al. (26) | TLR-9 | Indicative of the inflammatory response of breast cancer cells to BPs. | Decreased TLR-9 expression is associated with remarkably higher sensitivity to the growth-inhibitory properties of BPs. |
| Westbrook et al. (27) | CAPG and GIPC1 | Associated with subsequent development of bone metastases, reduced survival rate. Predictive of BP adjuvant therapy outcomes. | As a composite biomarker, the high expression of both proteins leads to a 10-folded increase in the ZOL effect. |
| Buranrat and Bootha (28) | MCF-7 | Induces cell proliferation- has a potential activity for metastasis | All BPs suppressed breast cancer MCF-7 cell progression by inhibiting cyclin D1 and inducing p21, caspase-3, and cytochrome c expression. |
| Hiraga et al. (29) | 4T1/luc | Responsible for migration and invasion of cancer cells | Zoledronic acid inhibited cell migration and invasion of 4T1/luc cells in a dose-dependent fashion |
Based on the fact that BPs play a pivotal role in breast cancer adjuvant therapy settings, Sandholm et al. (26) recognized Toll-like receptor 9 (TLR-9) as a functional biomarker for optimal BP administration. TLR-9 and nitrogen-containing bisphosphonates (n-BPs) are both capable of initiating a robust inflammatory response. Therefore, the authors assumed a possible correlation between TLR9 expression and cellular response to BPs. Based on the results of their study, breast cancer cells with decreased TLR9 were capable of sensitizing the growth-inhibitory features of BPs in vivo and in vitro, which suits TLR9 as a practical biomarker and indicator for optimal BP adjuvant therapy in breast cancer. Triple-negative breast cancer (TNBC) has been described as a poor-prognosis subtype of breast cancer. It has been revealed that low-TLR9 TNBC cells tend to respond to BP adjuvant therapy to a higher extent rather than high-TLR9 TNBC cells. The fact that non-nitrogen-containing BPs are considered anti-inflammatory while nitrogen-containing BPs are pro-inflammatory agents, explains why the findings were most pronounced with n-BPs. Again, Sandholm et al. (23) have recognized a cell protein entitled CD73 as another important biomarker in breast cancer associated with BP adjuvant therapy. Similar to TLR9, low-CD73 breast cancer tumors tend to benefit from adjuvant therapy with BPs. High CD73 expression is associated with cell invasion properties.
Seeking an unmet need for predictive and prognostic biomarkers of breast cancer, Westbrook et al. (27) identified and validated a composite biomarker entitled macrophage-capping protein (CAPG) and PDZ domain-containing protein (GIPC1), representative of the metastatic potential of breast cancer cells. In terms of CAPG and GIPC1 association with skeletal metastasis of breast cancer, this study revealed that high expressions of either CAPG or GIPC1 are indicative of greater risk of skeletal event development, and high expression of both biomarkers represents the highest risk for bone metastases in breast cancer patients. Although both of the biomarkers are capable of having independent prognostic potential for bone metastasis development, GIPC1 illustrates a stronger association with bone-only metastases of breast cancer. Moreover, bone metastasis as the first distant event is remarkably enhanced when both biomarkers represent high expressions, where ZOL can reduce the hazard of bone metastases in breast cancer patients to 90%. This amount reduces to only 9% when CAPG/GIPC1 expressions are low.
In a recent study by the same authors, Westbrook et al. (24) introduced another biomarker representative of high-risk bone recurrence in breast cancer patients. According to their study, dedicator of cytokinesis protein 4 (DOCK4) proved to be an appropriate biomarker for predicting response to ZOL adjuvant therapy. They suggested that DOCK4 has a similar predictive and prognostic value to CAPG and GIPC1 for skeletal-only relapses of breast cancer. Treatment with ZOL abolished the association of high levels of the aforementioned biomarkers in the development of skeletal-only metastasis. Moreover, Coleman et al. (25) identified the MAF biomarker as an important molecular goal for the treatment or prevention of bone metastases of breast cancer. MAF-negative, postmenopausal women benefit from ZOL adjuvant therapy in 80% of the cases while this treatment is suggested to be avoided in MAF-positive, not postmenopausal women as it may give rise to adverse disease outcomes. Consequently, MAF’s nuclear localization and absence of a catalyst domain make it a challenging pharmacological target. As stated by Westbrook et al. (24) the expression of both MAF and DOCK4 is induced by TGFβ and, therefore, DOCK4 expression correlates with MAF expression within the primary tumor. DOCK4 may also be an element of a protein panel that answers to high MAF expression within breast cancer cells which are bone-homing.
Buranrat and Bootha (28) in 2019 expressed the extent to which 3 Bisphosphonates decrease the viability of MCF-7 human breast cancer cells, stimulate cell apoptosis, and inhibit cell migration by changing proteins in the mevalonate pathway. They found that 3 Bisphosphonates made direct anticancer effects against MCF-7 cells in a dose and time-dependent way, with pamidronate demonstrating the highest efficacy. Besides, the BPs inhibited colony formation ability, and the activity of BPs against MCF-7 cells was prevented by the mevalonate product geranylgeranyl pyrophosphate, which was exacerbated by doxorubicin. They also showed that BPs exhibited a direct anticancer effect and an anti-migratory effect on MCF 7 cells. They suggested that BPs may be developed as an option for treatment of breast cancer and may serve as sensitizing chemotherapeutic drug.
Hiraga et al. (29) declared the effects of the BP ZOL, on visceral metastases of breast cancer, use of animal model in which mouse breast cancer cells 4T1/luc implanted at the mammary fat pad spontaneously metastasize to multiple organs including bone, lung, and liver in female BALB/c mice. Their results showed that ZOL has effects on breast cancer metastasis to visceral organs as well as bone. These effects of ZOL are inhibition of migration and invasion of breast cancer cells.
3.3. MRONJ in Breast Cancer Patients
In a recently implemented cross-sectional study by Soares et al. (11), 153 osteoporotic patients were compared with 134 metastatic breast cancer patients in terms of MRONJ prevalence due to the administration of oral and intravenous BPs. Metastatic breast cancer patients illustrated significantly lower levels of 25 hydroxyvitamin D (25OHD) and higher rates of procollagen1 amino-terminal propeptide (P1NP) in comparison with the osteoporotic group. Surprisingly none of the patients in the osteoporotic group were affected by MRONJ, whereas 4 cases (3%) of MRONJ were detected in the metastatic breast cancer group. Moreover, none of the biochemicals tested parameters including 25OHD, P1NP, osteocalcin, carboxy-terminal cross-linking telopeptide of type I collagen, intact parathyroid hormone, creatinine, and total calcium, were proved clinically useful for MRONJ risk assessment.
In a similar study by Tan and Barrett (30), 181 metastatic breast cancer patients were assessed in terms of osteonecrosis development and associated risk factors. The authors reported 13 patients with diagnosed MRONJ within a 4-year follow-up period; 12 patients with a history of IV BPs administration and 1 with denosumab after switching from BPs. The authors suggested a probable socio-economic profile for the disease as 6 out of 13 MRONJ patients were from deprived areas of Scotland. They accentuated the importance of counseling breast cancer patients about good oral and dental health, especially before their BP administration onset.
Patel et al. (31) have performed a study highlighting the key points of adjuvant BPs in early breast cancer and BRONJ risk. According to this study, females after menopause with intermediate-high risk early breast cancer get beneficial outcomes of adjuvant BPs in terms of mortality and cancer recurrence. As oral clodronate represents equal effectiveness as intravenous ZOL in the adjuvant therapy setting, it is safer to choose clodronate over ZOL due to its lower risk for BRONJ development. The risk of BRONJ continues long after adjuvant therapy cessation for more than 10 years and BRONJ risk increases with the duration of BP exposure at the same time; therefore, the termination of BP adjuvant therapy should be considered once the period of known benefits is completed (3 - 5 years). Finally, the authors emphasized the importance of pre-BP therapy dental assessments and BRONJ preventive measures and suggested that patients be equipped with Dental Alert Card for easier identification of patients at risk of BRONJ.
Matsuo et al. (32) evaluated dental implants as a risk factor for BRONJ in breast cancer patients. A 3-year study of 247 breast cancer patients with IV BP administration revealed a cumulative incidence rate of 0.074% for BRONJ development as only 1 out 6 breast cancer patients with dental implants was diagnosed with BRONJ. They concluded that dental implants, which were inserted before intravenous Bisphosphonate administration, were not a risk factor for the development of BRONJ in breast cancer patients.
MRONJ diagnosis process and its prevention play an important role on the quality of life of patients and the decision-making process by the majority of dentists involved in MRONJ prevention. A recent paper by Campisi et al. (33) reports the update of the conclusions from the Consensus conference, focused on the topic of MRONJ, and in particular on the common practices at risk of inappropriateness in MRONJ diagnosis and therapy, as well as on MRONJ prevention and the dental management of patients at risk of MRONJ. It is a matter of cancer and osteometabolic patients that are at risk since being exposed to several drugs with antiresorptive (bisphosphonates) or, more recently, antiangiogenic activities. The Conference was also traced for dentists and oral surgeons some easy applicable indications and procedures to reduce MRONJ onset risk and to diagnose it early. They stated that continuous updating on these issues, so important for the patient community, is recommended.
MRONJ has been reported as a side effect of bisphosphonate. In another study by Soares et al. (11), it has been reported that the prevalence of MRONJ was 3% in females with metastatic breast cancer receiving bisphosphonate. No cases were identified in women receiving oral bisphosphonate long term for osteoporosis. Procollagen type 1 amino-terminal propeptide was higher in females with metastatic breast cancer even during treatment with antiresorptive, but could not differentiate those with MRONJ.
Yu and Su (34) in 2020 investigated the therapeutic effect of various doses of teriparatide (TPTD) on BRONJ. They found that based on clinical and histomorphological observations, TPTD had a positive effect on treatment of BRONJ in a mouse model and administration of teriparatide had a beneficial effect on BRONJ in mice, but more studies are needed to determine whether the therapeutic effect on BRONJ is dose-dependent.
Hallmer et al. (35) in 2020 determined the incidence of risk factors of MRONJ in patients with metastatic breast cancer treated with ZOL and/or denosumab. They found that MRONJ developed in 4.1% of patients using ZOL during 77 months. Corticosteroid use was related to decreased risk of MRONJ, they claimed that diabetes was associated with an increased risk of MRONJ.
4. Discussion
Bisphosphonates have illustrated pivotal roles in various aspects of breast cancer treatment. However, the optimal use of them in different fields of breast cancer is still a matter of conjecture in terms of dosing, duration, drug selection, etc. Therefore, developing comprehensive and all-inclusive guidelines for bisphosphonates use in breast cancer patients is of great significance. Moreover, as we tried to infer, the biomarkers of breast cancer are appreciable tools for assessment, recognition, and evaluation of the optimal use of bisphosphonates in breast cancer patients, who has been barely noticed and investigated so far. Finally, a multidisciplinary approach between oncologists and oral health professionals is in demand before, during, and after BP consumption for the maximum control of osteonecrosis of the jaw as the most hazardous adverse events of these drugs. The method of use and also the duration of use of bisphosphonates play an important role in causing jaw necrotic lesions so that the risk increases in the injectable type and consumption of more than two years.
Previous literature indicates that bisphosphonates are used in patients with breast cancer who develop bone metastasis and are generally administrated every 4 weeks to lessen the risk of subsequent SREs. Also, bisphosphonates administration every 12 weeks is recommended in some guidelines. But, recently clinical trials suggested that bisphosphonate treats with reduced frequency (every 12 weeks) is not better than standard therapy. Yang and Yu (15) conducted an extensive study to demonstrate the efficacy and safety of these two treatment protocols. They reported that bisphosphonate administration every 12 weeks was not better in administration every 4 weeks. There was not any significant difference in SREs, renal dysfunction, and osteonecrosis of the jaw. In the exploratory experiment, patients who received intravenous bisphosphonates before the treatment experienced fewer on-study SREs, and a significant difference was observed between groups. Finally, they concluded that de-escalation treatment with bisphosphonates may be better than standard treatment in terms of efficacy, safety, and economic costs. But, it would be better that all the patients receive bisphosphonates every 4 weeks for several months before de-escalation.
According to Eguia et al. (16), All of bisphosphonates do not induce BRONJ. An increasing list of medications may have the same side effect with a higher/lower risk. Although much evidence does not exist for these drugs, it would be important to use clinical protocols that are similar to the ones used for patients administered bisphosphonates or denosumab. During next 2 to 3 years, it can be advised to treat, with special care, those patients treated with new biologic antiresorptive and anti-inflammatory agents and any other new antiangiogenic or immunosuppressive factors.
Adjuvant bisphosphonates can decrease breast cancer recurrence and death when given in a low-estrogen environment. Guidelines of treatment include recommendations for adjuvant bisphosphonates in postmenopausal patients. Gralow et al. (22) using 3 years of intravenous ZOL, oral clodronate, or oral ibandronate in patients with stage I-III breast cancer, have reported that osteonecrosis of the jaw was the highest for ZOL compared with clodronate and ibandronate. They didn’t found any differences in efficacy in the type of bisphosphonate, and in total analysis or subgroups. Despite an increased rate of osteonecrosis of the jaw with ZOL, rate of toxic reaction differed little across arms overally. It should be considered that patients performed a preference for the oral formulation, efforts to make oral drugs available in the United States should be made.
5. Conclusions
Bisphosphonates depicted remarkable advantages in improving SREs, SMR, survival rate, and treatment-emergent adverse events in breast cancer patients in almost all aspects of breast cancer therapy, It Includes adjuvant therapy for initial stages breast cancer to BMBC. The identification of breast cancer biomarkers that are capable of reflecting the outcomes of bisphosphonates therapy is a highly advantageous aid in the optimal utilization of these drugs. Breast cancer biomarkers such as MAF, DOCK4, CD73, TLR9, and CAPG/GIPC1 composite illustrated a significant correlation with bisphosphonates administration. MRONJ stands out as the most hazardous adverse event of the bisphosphonates with a rationally high incidence among breast cancer patients, which requires cautious prescription of bisphosphonates, as well as regular dental health counseling for being prevented. Bisphosphonates are great weapons in the arsenal of breast cancer treatment and, therefore, comprehensive studying of their features leads to the optimal and safe administration of them. Further investigations in terms of BPs function, related biomarkers, and MRONJ management/prevention in breast cancer patients are required.