1. Background
Invasive fungal infections (IFIs) are one of the main causes of mortality in patients with hematologic malignancy (1). The severity and duration of neutropenia, acute leukemia, the period following induction chemotherapy, allogeneic bone marrow transplantation (BMT), and functional disorders of lymphocytes are known as risk factors for invasive fungal infections (2, 3). Among mold infections, invasive aspergillosis (IA) is the most frequent IFI among hematologic malignancy patients following chemotherapy or BMT (4-6). Aspergillus spp. is a ubiquitous mold that is found on soil, rotten plants, and hospital surfaces which can enter the respiratory system through spores inhalation and cause serious diseases in susceptible people (1). Invasive sinopulmonary aspergillosis is a life-threatening fungal infection that usually affects neutropenic individuals (7, 8). Early diagnosis and timely treatment of the infection, improve the outcome of the disease (8). Definite diagnosis of IA is usually based on invasive techniques such as biopsy of the infected tissue and observation of the hyphal elements alongside angioinvasion or acquiring bronchoalveolar lavage (BAL) (4). However, early clinical and laboratory diagnosis of IA is a serious challenge and usually hampered due to overlapping and non-specific symptoms, coagulation problems, and special conditions of patients with leukemia (7, 9-11). Consequently, the infection rapidly progresses and the patients' outcome would be deteriorating (12). Over the past 30 years, 2 methods including computed tomography (CT) scan and serially checking of serum galactomannan (GM) have been effective in improving the IA diagnosis, although both methods have been helpful in detecting IA, none are pathognomonic (7, 11, 13). GM is an exo-antigen that is released when Aspergillus hyphae have been invaded by the patient's tissue and is usually shed into serum and BAL (6). Furthermore, 1,3-β-D-glucan is another noninvasive serum panfugal biomarker that is a typical cell wall component of most pathogenic fungi, such as Pneumocystis jirovecii and Candida spp., therefore, is less specific in detecting IA than GM (1, 3). Empirical approach and preemptive therapy are the main anti-fungal treatment approaches for neutropenic patients (12). Disadvantages of the empirical approach include increasing costs, raising the likelihood of resistance in microorganisms, and ultimately growing antifungal adverse effects (12). Preemptive therapy is the initiation of antifungal medicines when there is a non-invasive positive test such as a serum GM or an imaging index suggestive for fungal infection is evidenced, such as evidence of halo sign in chest CT scan (14). It has been suggested that even in leukemia patients who are at high risk for developing IA, the positive and negative results of serum GM should be cautiously interpreted regarding the probability of false results, and it is better to repeat these tests and clarify the results in consideration with clinical and radiological findings (7). Regarding the conditions of patients with hematologic malignancies and the limitation to perform many invasive diagnostic procedures due to the aforementioned reasons, we decided to investigate the role of nasal lavage fluid (NALF) GM level as a possible auxiliary method for IA diagnosis and compare the results with the levels of BAL GM in patients suspected of having the disease.
2. Methods
A prospective cohort study was conducted among adult patients with leukemia who were taking induction and/or consolidation chemotherapy in hematology ward of Imam-Khomeini Hospital complex in Tehran, Iran (September 2017 and August 2018) to investigate IA diagnosis via NALF GM assay in suspected patients. All patients with leukemia who presented febrile neutropenia (defined as a temperature of 38.5°C or more and absolute neutrophil count (ANC) < 0.5 × 109 cells/Liter) after chemotherapy and their fever did not cease within 4 to 6 days despite receiving empirical broad-spectrum antibiotics (carbapenem + vancomycin) along with negative blood cultures were included in the present study (15). A sample size of 32 subjects was statistically estimated to be sufficient to compare the diagnostic value of NALF GM with serum GM in suspected patients (with 80% power and a 5% level of statistical significance). All of the patients received fluconazole 400 mg daily as prophylaxis from the first day of chemotherapy. Amphotericin B deoxycholate 1mg/kg was started as empirical antifungal treatment in this phase. Before the first dose of amphotericin B, GM level of serum and NALF were assayed alongside mycological examinations for all of the patients with leukemia. To collect NALF specimens, the nasal cavity was irrigated by insertion of a soft catheter into each nostril (about 1 to 2 centimeters) and then 5 mL of normal saline was injected, the fluid of nasal lavage was collected in a sterile sink, then poured into a sterile container and sent to the mycological laboratory (15, 16). NALF samples were analyzed by direct microscopic examination using 10% potassium hydroxide (KOH) and culture. Processing of NALF samples was done under a high-efficiency particulate air-filtered hood. The specimens were inoculated onto fungal culture media named Sabouraud's dextrose agar (SDA) (Merck, Germany) and incubated at 30°C for 10 days. The grown fungi were identified through their macroscopic feature and microscopic morphology. The levels of GM were assayed using the Platellia Aspergillus enzyme immunoassay (EIA) kit (BIO-RAD, United Kingdom) according to the manufacturer’s recommendations. GM detection was done on each NALF and serum sample per subject by a mycologist who was blinded to case diagnoses and clinical conditions. Briefly, serum/ NALF samples were mixed well and 300 μL of each sample was added to 100 μL of 4% ethylene diamine tetra acetic acid (EDTA) treatment solution, boiled for 3 min, and subsequently centrifuged at 10,000 rpm for 10 min. fifty μL of each sample was added to the equal volume of a reaction mixture containing conjugated anti-GM EB-A2 antibody, and the mixture was incubated in microtiter plates precoated with the same antibody (EB-A2) for 90 min at 37°C. Wells were washed by an automated washer (SCO Diagnostics washer MPW1, Germany) and incubated with 200 μL of tetra methyl benzidine solution for 30 min in a dark environment. The reaction was stopped with 1.5 N sulfuric acid solution and the results were read under 450 and 620 nm optical densities (ODs). Positive, negative, and cutoff controls were in each assay. Results were documented as an index relative to the OD obtained from the threshold control (GM index = OD sample/OD threshold control). The results of GM level were regarded as positive when an OD index of 0.5 was achieved (6, 17). Ultimately, standardized Invasive Fungal Infections Group of the European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/ MSG) case definitions were used to categorize patients as having probable or possible IFI (18). The patients were considered to have proven IFI, if fungal hyphae alongside associated tissue damage were evidenced in the tissue specimen. Probable IFI was defined by the presence of at least 1 major or 2 minor clinical criteria for lower respiratory tract fungal disease or sinonasal fungal infections with mycological evidence of fungal infection (by cytology, direct microscopy, culture, or serum GM). Possible IFI was characterized by the presence of 1 major (or 2 minor) clinical criteria from abnormal site consistent with IFI but without microbiological support (18). Detection of GM in NALF was not considered as one of the microbiologic criteria of IFI.
2.1. Ethics Statement
The study was approved by the ethical committee of Tehran University of Medical Sciences (IR.TUMS.VCR.REC.1396.3180). In order to ensure anonymity, patients were assigned with numerical codes. All patients gave written informed consent based on ethical committee design before their inclusion in the study.
2.2. Statistical Analysis
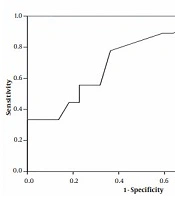
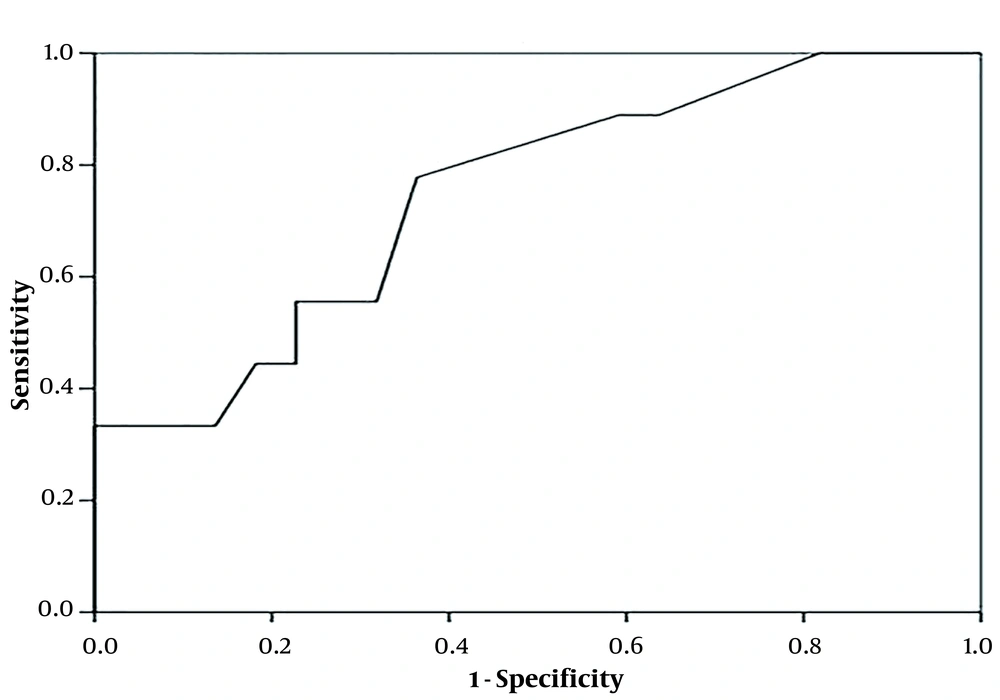
Sensitivity, specificity, negative predictive values, positive predictive values, likelihood ratios, diagnostic odds ratios, and k correlation along with their associated 95% confidence intervals (CIs), were estimated for NALF GM testing [at different optical densities (OD) cutoff values]. A receiver operating characteristic (ROC) curve was drawn up to evaluate how changing the OD index cutoff for GM EIA positivity affected sensitivity and specificity.
3. Results
Of 32 neutropenic patients with acute leukemia and possible, probable, or proven invasive fungal sinopulmonary infections, 14 (43.75%) patients had NALF GM ≥ 0.5 however, in 16 (50%) patients the level of serum GM was ≥ 0.5 (The The average age of the patients was 34 (range 14 - 63) years (Table 1). Direct examination of NALF was positive in 7 (21.8%) patients which all of them had positive NALF GM. Mycological cultures of NALF were positive in 9 (28%) patients (Aspergillus flavus grew on 7 media and Mucorales raised from 2 samples). Due to coagulation disorders, sinus endoscopy, bronchoscopy, and biopsy were only performed for 14 patients. Of these, in 6 patient's/biopsy specimens, whose NALF specimen showed a high level of GM (≥ 0.5), angular dichotomous and septated hyphae exhibiting aspergillosis were documented in the histopathological observations and consistently, cultures compatible with Aspergillus species were grown from all patient’s specimens (transbronchial biopsy or sinus tissue biopsy). Three out of 8 biopsy specimens from patients who had NALF GM < 0.5, showed positive histopathological examination and culture for Mucorales (2 of these 3 patients had positive NALF cultures) and 5 patients specimens remained negative for any molds. The level of NALFGM had a significant relation with the positive fungal culture and histopathological observation results of tissue biopsies (P = 0.048). The ROC curve demonstrated that GM levels of NALF with a cut-off of 0.45 had 78% sensitivity and 64% specificity for the diagnosis of IA (P = 0.033) (Table 2 and Figure 1). Among the serum GM positive patients, 71.4% had NALF GM ≥ 0.5. Interestingly, 2 patients with probable invasive fungal infection had a serum GM level of less than 0.5, but the GM of NALF was positive and IA was eventually confirmed by tissue biopsy in both.
| Patients | Proven IA (n = 6) | Probable IA (n = 12) | Proven Mucormycosis (n = 3) | Non-IFI (n = 11) | Total (n = 32) |
|---|---|---|---|---|---|
| Female/male | 3/3 | 4/8 | 1/2 | 3/8 | 11/21 |
| AML (M3: 1 Non-M3:14) | 4 | 7 | 2 | 2 | 15 |
| ALL | 2 | 5 | 1 | 9 | 17 |
| Positive microscopy/culture showing Aspergillus or Mucorales of NALF specimens | 6/5 | 0/2 | 1/2 | 0 | 7/9 |
| Positive histopathologic examination/ positive culture | 6/6 | 0/0 | 3/2 | 0 | 9/8 |
| Serum GM ≥ 0.5 index | 6 | 10 | 0 | 0 | 16 |
| NALF GM ≥ 0.5 index | 6 | 8 | 0 | 0 | 14 |
| Simultaneously elevated serum and NALF GM | 6 | 6 | 0 | 0 | 12 |
Abbreviations: IFI, invasive fungal infection; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; NALF, nasal lavage fluid; GM, galactomannan.
| Cut-off Point of NALF-GM | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| 0.45 ≤ | 78 | 64 | 47 | 87 |
| 1 ≤ | 33 | 86 | 5 | 76 |
Abbreviations: NALF, nasal lavage fluid; PPV, positive predict value; NPV, negative predict value.
4. Discussion
Chemotherapy is currently the standard treatment for patients with hematological malignancies, and invasive fungal infections are one of the most common adverse complications in this setting (19). IFIs are mostly prevalent in patients with acute leukemia, especially after induction chemotherapy (5). In one study on patients with hematological malignancies, the prevalence of IFIs was highest in patients with acute myeloid leukemia (AML), and A. flavus with 44% was the most common cause (19). In this study with 17 patients with acute lymphoblastic leukemia (ALL) and 15 AML patients, the prevalence of proven IFIs was higher in patients with AML; however, this difference was not statistically significant. In addition, the most common causal agent was A. flavus. Despite extensive studies, there are still serious controversial topics about the best way to diagnose IFIs, especially when IA is suspected in patients undergoing chemotherapy or BMT (18). Given the low sensitivity of mycological culture to detect aspergillosis, relying on culture alone is not enough to rule out the infection and even new methods such as polymerase chain reaction (PCR) are not superior to cultures for detecting invasive mold infections (18, 20). IA in the patients with hematologic malignancies usually causes sinopulmonary involvement because their immunocompromised status may provide an opportunity for growing spores and invade sinonasal mucosal tissue. Therefore, sampling from this infected tissue may supply a quick and accessible diagnostic method (3, 21). One of the important diagnostic specimens in these infections is sinonasal endoscopy, where the existence of pale and necrotic mucosa would be strongly favorable for IFI. Biopsy of the involved areas for pathological and mycological examination could be effective in getting a definitive diagnosis, although in many cases due to coagulation disorders such as severe thrombocytopenia, this procedure cannot be feasible in the acute phase of the disease. For the first time, the nasal lavage method was explained as a diagnostic measure by Hirvonen et al. in 1999 (15). The nasal lavage technique is a non-invasive procedure, well-tolerated, and easy to perform. In some studies, NALF has been used for the diagnosis of chronic rhinosinusitis and evaluation of the response to topical steroids therapy in non-allergic rhinitis with eosinophilia (16, 22). To the best of our knowledge, the mentioned study is the first study investigating the diagnostic value of NALF in the detection of IFIs, especially IA through its GM assay in patients with leukemia. Samples taken from sterile fluids are much more valuable for the diagnosis of IFIs than those obtained from non-sterile sites that are likely to be colonized (BAL, sinuses, respiratory secretions, and the like) (18). Finding fungal elements in non-sterile secretions can be contributory for detection of an IFI if there are related predisposing factors along with clinical and/or radiological evidence, but it is not confirmatory (18). In a study on 58 BAL specimens obtained from hematological malignant patients with suspected pulmonary fungal infections, 29 (50%) mycological cultures were positive, however, fungal hyphae were demonstrated in 23 mycological smears (10). NALF used in the current study, was a non-sterile and accessible fluid which collected from 32 patients with hematological malignancies, and showed positive mycological smear and culture in 7 (21.8%) patients and 9 (28%) patients, respectively. CT scans and serological testes such as GM assay are being frequently used for diagnosis of invasive sinopulmonary fungal infections, especially IA (23). There are currently many leukemia centers around the world that apply the imaging modalities especially thoracic CT scan as the first diagnostic tool guiding for anti-fungal treatment initiation, however, considering the poor specificity of lung CT scan to diagnose invasive lung aspergillosis, the result of imaging patterns should be interpreted alongside mycological and serological tests (10, 24). Many cut-off values have been reported in various studies in order to encourage sufficient sensitivity and specificity of serum GM for IA diagnosis, for example in a study on allogeneic hematopoietic stem cell transplant recipients for early detection of IA, the sensitivity and specificity were about 94% and 99%, respectively (13). Although previous studies showed high sensitivity and specificity of serum GM tests, subsequent prospective studies demonstrated a specificity of 85 - 99% and a wide range of sensitivity of 29 - 94% for GM tests on patients with hematologic malignancies (17). BAL GM cut-off also is a challenging topic, for instance in a study on hematological malignant patients, using a BAL GM cutoff OD = 1.1 was associated with IPA detection with 100% sensitivity and 98% specificity (8). In another study of patients with hematologic malignancy who were suspected to have IA, the sensitivity of BAL GM assay was 91% (with a cut off OD ≥ 1) and in comparison with the mycological BAL culture which showed 50% sensitivity, BAL GM was a significantly better test (10). GM levels in fragments of the specimens removed from the sinus endoscopy have also been investigated in immunocompetent patients with fungal rhinosinusitis that the sensitivity and specificity were 87 and 88%, respectively (25). In a study conducted by Kostamo et al. on GM levels in mucosal sinus specimens and NALF in patients with chronic rhinosinusitis, there were 5 (20%) positive samples in the patient and 4 (21%) positive samples in the control group (with cut-off ≤ 1) and it was concluded that NALF GM test is not reliable for the diagnosis of chronic Aspergillus rhinosinusitis (26). In the current study with NALF GM cut-off ≥ 0.5, a sensitivity of about 78% and specificity of 64% was demonstrated for the diagnosis of IA in the patients with leukemic. The use of piperacillin tazobactam is the only variable that significantly associated with the false positive results of GM (7). In this study, none of the patients received piperacillin tazobactam. Many centers use anti mold azoles or other antifungal agents as prophylaxis that could affect the results of serum GM level (14), but none of our patients were taken anti mold prophylaxis. According to the studies, there is no significant relationship between serum GM levels and the extent of lung involvement in aspergillosis (17). No comparisons were made between radiological evidence and serum or NALF GM in the current study.
4.1. Conclusion
Beside other tests, mycological indexes specially GM level of NALF would be likely useful to diagnose IA in patients with leukemia as a safe and accessible diagnostic method. Further studies are needed to confirm this test as a new index for antifungal preemptive therapy in these patients.