1. Background
Glands in the human body are responsible for secreting hormones, enzymes, and other various substances. When the cells of these normal glands grow out of control, they are called adenocarcinoma. Gastric adenocarcinoma is the most common type of gastric cancer, which is prevalent in 95% of patients with gastric cancer (1, 2). Gastric cancer is the 5th leading cause of cancer and the 3rd main cause of cancer deaths, accounting for 7% of all cancers and 9% of cancer deaths. In 2012, 950000 people worldwide had this type of cancer, which killed 723000 people (3). According to the latest reports from the Ministry of Health, adenocarcinoma in Iran is considered to be the deadliest type of gastric cancer, and people with this type of cancer have a shorter life expectancy than other types of gastric cancer (4). Adenocarcinoma is the most common pathological type of cancer (5). Recent studies have shown a relationship between metabolic syndrome and increased risk of gastric adenocarcinoma (6).
In standard survival data, only one event is expected, and individuals experience only one event until the end of the study. Many studies of survival factors in gastric cancer patients with only one cause of death have been reported, in which standard survival methods to evaluate survival, including Kaplan-Meier (KM), log-rank test, and Cox or parametric regression models have been used (7-10). In some survival studies, there may be several occurrences of interest to the researcher, some of which are the terminal causes of the event; following their occurrence, the follow-up of patients at the time of this type of event will end (such as death due to the progression or due to other causes). Some of the causes of events are non-terminal (such as local recurrence or distant recurrence); following their occurrence, follow-up will continue until the patient experiences the terminal event or will be censored at the end of the study. In this case, semi-competing risks are used to analyze these types of survival data (11, 12). Factors affecting relapse and death in patients with gastric cancer have been evaluated in the presence of semi-competing risks with different models (13, 14).
However, in many medical studies, there may be several causes for each individual to end the study, and all causes are terminal events (such as death due to disease progression or death due to other causes). And only the time and type of the first event for each individual should be recorded, and the other events should be considered "competing events"; this type of multiple events is called competing risks. As the analysis of this type of data using conventional methods of survival analysis, including KM or log-rank in the presence of competing risks, leads to an overestimation of the hazard, appropriate methods should be used. In this regard, 2 commonly-used methods of analysis of competing risk include cause-specific hazard and subdistribution hazard regression (15, 16).
Buzzoni et al. (2015) performed a study on gastric adenocarcinoma patients that randomly divided into chemotherapy and no chemotherapy groups to compare the cumulative incidence function (CIF) of local recurrence in the two groups, in which distant metastatic events, secondary malignancies, and death were considered competing events. Also in this study, the incidence of local recurrence, secondary malignancies, and death was considered competing risks to compare the incidence of distant metastasis in the two groups (17).
In a study conducted by Kim et al., on patients with gastric and esophageal cancer, the incidence and mortality of each cancer were assessed, and mortality due to cancer-unrelated causes was considered a competing event in each cancer. In this study, the Cox proportional hazard (PH) model was used to evaluate the effects of covariates and, if the PH was not assumed, a logistic regression model for specific times would be used to evaluate covariates on cancer-related death (18).
Kubota et al. assessed the survival and its associated factors in gastric cancer in the presence of competing risks. In their study, death due to cancer was considered the interest event, and death due to other causes was considered the competing risk (19).
Kim et al. (2016) investigated the effect of eradication of Helicobacter pylori on long-term survival after distal gastrectomy in patients with gastric cancer. In this study, peritoneal recurrence was considered an interest event, and local, regional, distant, and death events were considered competing events (20).
In a study by Kubata et al. (2014), they examined the prognostic significance of postoperative complications in gastric cancer patients. In their study, the subdistribution regression model was used for cancer-specific mortality and death because other causes were considered a competing event (19).
A study by Strong et al. (2013) compared disease-specific survival in the United States and Korea after resection for node-negative early gastric cancer. To estimate and compare the cumulative rate of disease-specific mortality, mortality due to other causes or unknown causes were considered competing events. In this study, the multivariate regression model was used to assess the effect of the country on disease-specific survival after controlling for important prognostic factors (21).
In another study, Strong et al. (2015) compared the survival of gastric cancer patients between the United States and China. In his study, death due to cancer was considered to be the interest event, and death due to other causes was considered a competing risk. They used multivariate competing risk analysis to assess risk factors for death due to cancer (22).
Thus, given that there are two common regression models in the presence of competing risks for modeling the effect of covariates on the hazard of the outcome or modeling the effect of covariates on the cumulative incidence function. The first one is the cause-specific hazard regression model, which allows estimating the effect of the covariates on the rate of occurrence of the outcome in those subjects, who are currently event-free, and also it is better suited for addressing etiologic questions. The second one is the subdistribution regression hazard model, which allows estimating the effect of covariates on the absolute risk of the outcome over time and is better suited for estimating a patient’s clinical prognosis (15, 16). Also, a previous study identified risk factors for death in gastric patients without considering the cause of death and used classical survival methods.
2. Objectives
This study aimed at evaluating the factors affecting the hazard of death due to cancer-related with taking into account competing events in patients with gastric adenocarcinoma using cause-specific and subdistribution hazards regression models.
3. Methods
In this study, 306 patients with gastric adenocarcinoma at Imam Khomeini clinic were studied in Hamadan Province, in the west of Iran, from 2001 to 2017. The collected data included demographic characteristics, including age at diagnosis, gender, clinical and pathological characteristics, tumor differentiation, stage of the disease, and treatment [surgery, chemotherapy (CT), radiotherapy (RT)]. The survival status of patients was followed up, using patient referrals and telephone calls.
Follow-up was continued from the date of diagnosis to the date of death or up to the end of the study. Gastric cancer-specific survival was defined as the date of diagnosis to the date of death due to cancer progression cause. All patients who lost the follow-up (because of migration, missing or changing in contact information, and so on), as well as patients who remained alive at the end of the study in December 2017, were considered censored. Also, patients who experienced competing events (in this study died without cancer progression or unknown causes) were coded as censored cases, too. This study was approved by Hamadan University of Medical Sciences (code: project number 9711237184 with the specific Ethics ID code IR.UMSHA.REC.1397.835).
Survival times were calculated from the date of diagnosis till death due to cancer progression or other causes, and patients who did not die by the end of the study were considered censored. The primary interest outcome in this study was death after progression to cancer metastasis (considered cancer-related death) and patients' death without progression of disease was considered a competing event (considered unrelated cancer death); 162 patients died by the end of the study (115 patients died after cancer progression and 47 patients without metastasis).
3.1. Statistical analysis
The incidence of death due to cancer progression and other causes was estimated, using CIF. Overall survival was compared with the log-rank test. The log-rank test is a large-sample chi-square test that provides an overall comparison of the KM curves (23). Crude cumulative incidence curves (CICs) were estimated in categories of each covariate, using CIF, and compared using the Gray test that is analogous to the log-rank test (24). The cause-specific hazards regression model was used to assess risk factors on instantaneous hazard and the subdistribution hazard regression model developed by Fine and Gray was applied to estimate the hazard ratio for cumulative incidence mortality (25). All analyses were conducted, using R3.6.1 software and cmprsk and survival packages. P-value < 0.05 was set statistically significant.
4. Results
The mean (standard deviation) and the median age of patients were 62.3 (12.5) and 63 years (range, 24 - 92 years); 234 (76.5%) patients were male. Patient characteristics and comparison of survival and cumulative incidence of death due to cancer progression were presented in Table 1.
| Variables | No. (%) | Cancer-Related Death | Other Cause of Death | Log-rank Test | Gray Test |
|---|---|---|---|---|---|
| Gender | 0.6 | 0.55 | |||
| Male | 234 (76.5) | 87 | 38 | ||
| Female | 72 (23.5) | 28 | 9 | ||
| Age at diagnosis | 0.4 | 0.62 | |||
| ≤ 50 | 53 (17.3) | 21 | 5 | ||
| 51 - 75 | 214 (69.9) | 76 | 31 | ||
| > 75 | 39 (12.7) | 18 | 11 | ||
| Tumor grade | 0.2 | 0.1 | |||
| Good | 27 (8.8) | 9 | 4 | ||
| Moderate | 69 (25.5) | 22 | 11 | ||
| Poor | 74 (24.2) | 29 | 12 | ||
| Unknown | 136 (47.5) | 1 | 4 | ||
| Metastasis location | < 0.01 | < 0.01 | |||
| Liver | 69 (22.5) | 47 | 0 | ||
| Other | 40 (13.1) | 26 | 0 | ||
| Unknown | 197 (64.4) | 42 | 47 | ||
| Type of treatment | < 0.01 | 0.02 | |||
| CT | 127 (44.9) | 52 | 18 | ||
| Surgery | 7 (2.5) | 4 | 0 | ||
| CT & RT | 24 (8.5) | 5 | 7 | ||
| CT & Surgery | 94 (33.3) | 33 | 10 | ||
| All three | 31 (11) | 6 | 7 | ||
| Surgery type | 0.03 | 0.17 | |||
| Total | 51 (38.9) | 17 | 6 | ||
| Subtotal | 31 (23.7) | 8 | 8 | ||
| Partial or distal | 49 (37.4) | 13 | 4 | ||
| Disease stage | < 0.01 | 0.035 | |||
| 1 - 3 | 45 (14.7) | 0 | 16 | ||
| 4 | 181 (59.2) | 115 | 0 | ||
| Unknown | 80 (26.1) | 0 | 31 | ||
| Tumor location | 0.3 | 0.54 | |||
| Cardia | 40 (13.1) | 15 | 7 | ||
| Body | 41 (13.4) | 17 | 8 | ||
| Antrum | 38 (12.4) | 13 | 10 | ||
| Other | 23 (7.5) | 19 | 3 | ||
| Unknown | 164 (53.5) | 51 | 19 | ||
| Involved lymphoma | < 0.01 | < 0.01 | |||
| ≤ 2 | 55 (18) | 12 | 12 | ||
| > 2 | 46 (15) | 15 | 8 | ||
| Unknown | 205 (67) | 88 | 27 |
Characteristics of Gastric Cancer Patients with Adenocarcinoma Tumor and Comparison of Deaths by Different Causes a
The results of the log-rank test showed that the location of metastasis, type of treatment, surgery type, stage, and involved lymphoma had a significant effect on survival (Table 1). Also, based on the Gray test, the difference of cumulative incidence of mortality was statistically significant in the levels of the location of metastasis, type of treatment, stage, and involved lymphoma.
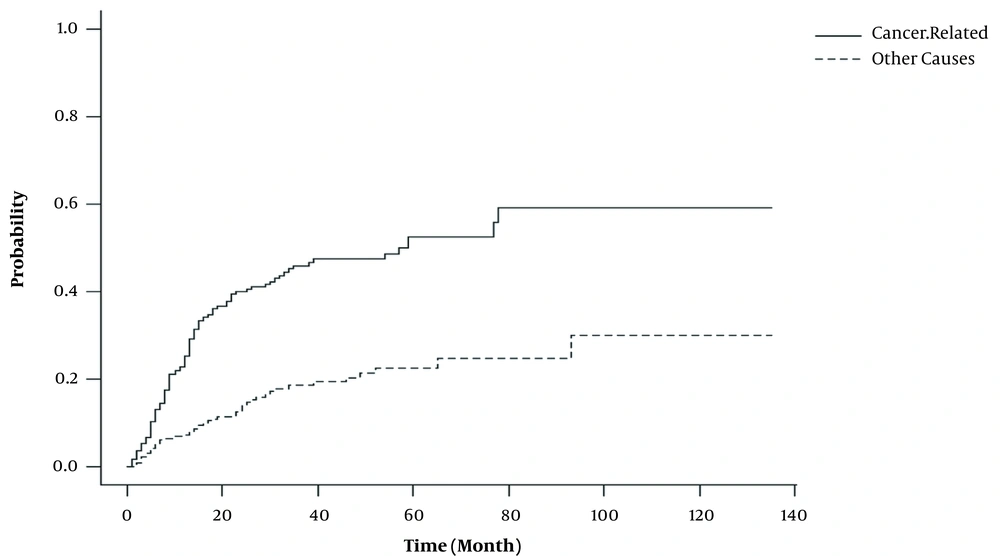
Figure 1 showed that the mortality risk due to cancer progression was higher than other causes of death. Up to 65% of patients experienced death related to progression over 3 years after diagnosis, 46% of whom were due to progression of cancer and 19% were due to other cause. Regarding the relationship between survival and CIF, survival probability was estimated for 1, 2, 3, 4, 5, and 10 years as presented in Table 2.
| Time (y) | CIFCancer-Related | CIFOther-Cause | CIFTotal | Survival (1-CIFTotal) |
|---|---|---|---|---|
| 1 | 0.25 (0.2, 0.3) | 0.08 (0.04, 0.1) | 0.33 (0.26, 0.38) | 0.67 (0.62, 0.74) |
| 2 | 0.4 (0.34, 0.46) | 0.14 (0.1, 0.19) | 0.54 (0.46, 0.62) | 0.46 (0.38, 0.53) |
| 3 | 0.46 (0.39, 0.53) | 0.19 (0.13, 0.24) | 0.65 (0.56, 0.73) | 0.35 (0.27, 0.44) |
| 4 | 0.47 (0.41, 0.54) | 0.21 (0.15, 0.26) | 0.68 (0.6, 0.77) | 0.32 (0.23, 0.41) |
| 5 | 0.53 (0.45, 0.6) | 0.23 (0.16, 0.29) | 0.76 (0.65, 0.85) | 0.24 (0.15, 0.35) |
| 10 | 0.59 (0.48, 0.69) | 0.3 (0.17, 0.43) | 0.89 (0.72, 1) | 0.11 (0, 0.28) |
Estimation of 1, 2, 3, 4, 5, and 10-years Cumulative Incidence (95% CI) and Survival Probability (95% CI) in Adenocarcinoma Gastric Cancer Patients, Using CIF in Difference Competing Event
Using cause-specific and subdistribution hazards regression models, risk factors for cancer progression in the presence of competing risks were estimated. The results of both regression models are presented in Table 3.
| Variables | Subdistribution | Cause-Specific Hazard Ratio (95% CI) | ||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | P-Value | Hazard Ratio (95% CI) | P-Value | |
| Gender | ||||
| Female | 1 | 1 | ||
| Male | 1.14 (0.74 - 1.77) | 0.55 | 1.12 (0.73 - 1.72) | 0.54 |
| Age at diagnosis | ||||
| ≤ 50 | 1 | 1 | ||
| 51 - 75 | 0.96 (0.59 - 1.55) | 0.86 | 1.04 (0.64 - 1.68) | 0.37 |
| > 75 | 1.4 (0.64 - 2.27) | 0.57 | 1.48 (0.79 - 2.79) | 0.85 |
| Cancer grade at diagnosis | ||||
| Well differentiated | 1 | 1 | ||
| Moderately differentiated | 0.82 (0.37 - 1.83) | 0.87 | 0.74 (0.34 - 1.64) | 0.43 |
| Poorly differentiated | 1.06 (0.49 - 2.27) | 0.66 | 1.03 (0.49 - 2.18) | 0.49 |
| Undifferentiated | 0.45 (0.1 - 2.16) | 0.87 | 0.48 (0.1 - 2.22) | 0.68 |
| Treatment type | ||||
| CT | 1 | 1 | ||
| CT & RT | 0.36 (0.14 - 0.89)* | 0.03 | 0.33 (0.13 - 0.83)* | 0.03 |
| CT & Surgery | 0.8 (0.54 - 1.2) | 0.28 | 0.74 (0.48 - 1.13) | 0.16 |
| CT & RT & surgery | 0.29 (0.13 - 0.66)* | 0.003 | 0.25 (0.11 - 0.58)* | 0.01 |
| Surgery type | ||||
| Total | 1 | 1 | ||
| Subtotal | 0.66 (0.3, 1.45) | 0.3 | 0.7 (0.3, 1.6) | 0.15 |
| Partial or distal | 0.82 (0.42, 1.61) | 0.56 | 0.78 (0.88, 2.53) | 0.25 |
| Tumor location | ||||
| Cardia | 1 | 1 | ||
| Body | 1.27 (0.66, 2.45) | 0.47 | 1.38 (0.69, 2.77) | 0.18 |
| Antrum | 0.82 (0.39, 1.71) | 0.59 | 0.82 (0.39, 1.72) | 0.67 |
| Other | 1.12 (0.6, 2.12) | 0.71 | 1.04 (0.52, 2.05) | 0.88 |
| Involved lymphoma | ||||
| ≤ 2 | 1 | 1 | ||
| > 2 | 1.52 (1.09, 3.14) * | 0.04 | 1.48 (1.15, 4.18)* | 0.02 |
| Unknown | 2.91 (1.63,5.19)* | 0.001 | 3.1 (1.69, 5.66)* | 0.001 |
| Disease stage | ||||
| 1 - 3 | 1 | 1 | ||
| 4 | 2.9 (1.7, 6.8)* | 0.001 | 2.2 (1.6 - 6.3)* | 0.001 |
Evaluation of Risk Factors on Death Due to Cancer Progression in Gastric Cancer Patients with Adenocarcinoma Tumor in the Presence of Competing Risks Using Subdistribution and Cause-Specific Hazard Regression Model
The results in Table 3 showed that in both models, tumor stage, type of treatment, and the number of involved lymph nodes were a significant risk factor in the hazard of death due to cancer progression. Patients with all treatments (CT & RT & surgery) considerably reduced the hazard of death. In patients with more than 2 involved lymph nodes, the hazard of death was 1.5 times higher, and the mortality hazard in patients with stage 4 was more than 2 times lower.
5. Discussion
The current study aimed at evaluating factors affecting the hazard of death in patients with gastric adenocarcinoma, who died from cancer progression in the presence of other competing mortality risks, using cause-specific and subdistribution hazards models. As in other studies, in this study, the ratio of women to men was 1/3, and although the hazard ratio of cancer-related death in men was higher in both regression models, the effect of gender was not significant on the hazard of death due to cancer progression similar to other studies (9, 26, 27).
Mean (SD) age at diagnosis was 62.3 (12.5) years old (range: min = 24 and max = 92). Consistent with other studies, as the age at diagnosis increases, the hazard of death increases (9, 13) but in the current study its effect was not significant on the hazard of death.
In this study, 1, 3, and 5 survival rates in the presence of competing risks were 67.4. 35.4 and 24.9%, respectively, which have been estimated more than other studies (8, 9, 26-28). This may be because the Kaplan-Meier curve underestimates the survival probability in the presence of competing risks. Therefore, in this study, the CIF was used to estimate the survival rate, in which the underestimation problem obtained by Kaplan-Meier seems to be resolved.
As expected, the results of our study showed that the mortality risk from cancer-related was higher than the other causes (Figure 1). Also, as depicted in Table 2 and Figure 1, the most incidence of death occurred within the first 3 years of follow-up (about 46% of death due to cancer-related and 19% other causes); likewise, the 5-years cumulative incidence of cancer-related mortality (due to cancer progression and other causes) was 52.5 and 22.6%, respectively. Strong et al. showed that the cumulative incidence of cancer-related death in Chinese patients was 53% (22). The similarity of these two studies may be due to the similarity of demographic and clinical characteristics. The cumulative incidence of 5-years mortality due to other causes in Chinese patients was 2%, which is different from the present study (22). The reason for this difference may be because, in the present study, patients whose cause of death was unclear were considered to have passed away due to other causes. In the other study on the United States, the 5-years incidence of cancer-related and other causes of death was 32 and 10%, respectively, which is significantly different from the present study (22). The difference between these findings may be due to the clinical and demographical characteristics affecting the cumulative incidence in these two studies. Although the median age of diagnosis in the present study was lower than that of the American patients (63 vs. 69 years), in the present study, this variable was not effective and many clinical risk factors influencing survival in this study significantly differed from American patients. In addition, the majority of patients were diagnosed in advanced stages of the disease (almost 59.2% of patients diagnosed with stage IV disease), but in the American patients, just 5% of the patients were diagnosed in stage IV (22).
In the present study, the cumulative incidence of 10-year cancer-related mortality was 89%. In Morais et al.’s (2017) study in Portugal with primary gastric cancer patients, the 10-year cumulative incidence of death was 69.5% (29). Also, in another study by Morais et al. (2018), the 10-year cumulative incidence of mortality in second primary cancer patients was about 56% (30). The high 10-years incidence of mortality in this study has pertained to late diagnosis of disease, high stage of the tumor along with metastasis to other organs, and ineffectiveness of treatment in this situation.
In this study, the use of complementary therapies had a significant effect on reducing the risk of death due to cancer progression, in which the risk of death reduced more than 70% in patients receiving all treatments (surgery, radiotherapy, and chemotherapy). In two meta-analysis studies aimed at summarizing the effects of treatment on the survival of patients with gastric cancer, the combination of treatments was effective and reduced the risk of death by 20 to 30%. Other studies have also confirmed these results (8-10, 26, 31, 32). In the study of Zhang et al. (2019), the effect of surgery, chemotherapy, and chemo-radiotherapy on the risk of mortality from cancer progression in gastric adenocarcinoma patients based on a subdistribution model was significant (33). Sun et al. (2019) conducted the mortality risk in patients who were not treated with surgery and chemotherapy increased 1.6 and 2.5 times, respectively. Although the risk of death increased in patients who did not receive radiotherapy, this increase was not significant (34). The effectiveness of the first treatment in reducing mortality risk in the present study compared to other studies could be due to the majority of patients receiving complementary treatments. As regards, 98% of the patients in this study received chemotherapy and about 50% received surgery. Also, more than 50% of patients in the present study received at least two types of treatment.
In the present study, because of cancer progression, the number of involved lymphomas had a significant effect on the hazard of death, and as the number of involved lymphomas increased, the hazard of death significantly increased. In most studies, this variable was identified as an independent risk factor (19, 26, 28, 35). As the disease stage and metastasis to other organs are associated with lymph node involvement, the higher disease stage affected higher involved lymph nodes. As shown in the descriptive results of this study, the majority of patients were diagnosed in advanced stages of the disease; so, the effect of this factor on the risk of death due to cancer progression was not unexpected.
The stage in both the cause-specific and subdistribution models had a significant effect on the risk of death due to cancer, so the risk of death in stage 4 was more than twice that of stage 2 - 3. In Kim's study (2016), the risk of death in stage 4 was more than 9 times (18). Also in the study of Hamashima (2015), the disease stage considerably increased the risk of death due to the progression of gastric cancer (36).
Other variables in this study had no significant effect on mortality hazards. Although the hazard of death was different in levels of risk factors in both methods (Table 3), these differences were not statistically significant in the presence of other variables.
Due to the retrospective nature of this study, the first limitation was the incompleteness of some information such as pathology reports and history sheets for some patients. And the second was the unknown recording of the causes of death or even multiple causes of death for some patients due to lack of contact information or changing the phone number might affect the analysis results.
5.1. Conclusions
If both competing risk models indicate a significant association between covariates and the hazard, as in this study, there is a real effect between covariate and hazard of interest event. But, if the two models provide different results, the researchers should specify the research goals. In general, the subdistribution hazard is most suitable for the prediction of a survival probability, while the cause-specific approach is appropriate for etiological studies.