1. Background
Head and neck cancers are quite prevalent, and the majority of patients present with locally advanced disease. Other than surgery, radiotherapy concomitant with chemotherapy (CRT) is the standard of care in the case of locally advanced head and neck malignancy (1). Acute side effects such as mucositis, dysphagia, and subsequent weight loss are the most critical morbidities and dose-limiting complications in patients undergoing CRT. Severe mucositis and its associated morbidities can lead to an interruption in RT treatments. Thus, it may have a potential negative impact on survival (2). The incidence of radiation-induced mucositis in head and neck cancer patients reaches up to 80%. The rate of grade 3 and 4 mucositis is 56% in patients who received altered fractionation radiotherapy, while the incidence decreased down to 34% in patients undergoing conventional methods (2, 3).
Cyclo-oxygenase 2 (COX-2), an enzyme induced in pathological states, mediates the production of prostaglandins (PG) (4). In several tumors, overexpression of COX-2 correlates with aggressive behavior, poor prognosis, and development of metastatic disease. In general, about 70% of squamous cell carcinomas of the head and neck have COX-2 overexpression (5). In submucosal tissues, COX-2 expression elevates in response to radiation. This expression contributes to the development of ulcerative mucositis (6). The use of a COX-2 inhibitor may delay tumor growth and decrease radiation-induced toxicity in healthy tissues. These observations may suggest therapeutic gain with radiotherapy combined with COX-2 inhibition (4). Meanwhile, in animal studies, the administration of celecoxib as a COX-2 inhibitor has demonstrated a dramatic nephroprotective effect against the cisplatin-induced renal injury (7).
2. Objectives
This study is a double-blinded randomized placebo-controlled clinical trial (RCT) for evaluation of a COX-2 inhibitor (celecoxib) concurrently with chemoradiation as a treatment modality for locally advanced head and neck carcinomas.
3. Methods
Our study was a double-blinded, randomized, placebo-controlled clinical trial. The study was conducted in outpatient setting in the radiation oncology ward (Cancer Institute, Tehran, Iran). The institutional review board and the ethics committee of the vice-chancellor of research of Tehran University of Medical Sciences approved the study design. The study design and goal were disclosed to the eligible patients to obtain written informed consent before randomization. The study design was in line with the ethical guidelines of the Declaration of Helsinki. Patients were randomized into two groups (celecoxib and placebo) assigned by the permuted blocks method. The patients and the physicians, who evaluated the side effects, were blinded to the assignment. The protocol of the study was registered and verified in the inventory of clinical trials accessible at clinicaltrials.gov (NCT00603759).
3.1. Inclusion Criteria
We recruited patients with locally advanced head and neck carcinomas (T3-T4 or N+ve, according to the 6th edition of the AJCC staging system) between 2006 and 2008. The tumor location was in any of the nasopharynx and oral cavity, oropharynx, or hypopharynx, or larynx. The squamous cell carcinoma patients with affected cervical lymph nodes of an unknown primary site in the head and neck region were also enrolled. In the case of laryngeal tumors, only the patients with supraglottic involvement that required elective level 1b irradiation was included. It was needed for all the patients that at least 50% of the oral cavity should be in the radiotherapy field.
3.2. Exclusion Criteria
We excluded patients who had less than 18 years of age, or Karnofsky performance status (KPS) < 70, or with distant metastasis (M1). We also excluded those with a known hypersensitivity to COX-2 inhibitors or non-steroidal anti-inflammatory drugs (NSAIDs) and with a history of chronic use of NSAIDs or corticosteroids (continuous consumption of these medications to at least one month before the study). Individuals who previously received radiation to the head and neck region or surgeries in this area were kept out of the study.
3.3. Treatment Protocol
The patients were to receive a total radiation dose of 66-70 Gy (in 2 Gy per fraction, 5 days a week) through the conventional radiotherapy technique via the Cobalt 60 external beam radiotherapy machine. The treatment was planned with the X-ray simulation method. The volumes of treatment comprised 3 phases as follows: the first gross tumor and areas of microscopic extension and elective neck nodes with 1 cm margin; the second phase consisted of gross tumor and areas of microscopic extension alone with 1 cm margin; the third phase covered gross tumor with 5 mm margin. The coverage of elective nodes was based on the location of the primary tumor, its extension, and the level of involved neck nodes. In the case of the supraglottic tumors extending toward the base of the tongue, we covered level Ib neck nodes that mandated radiating “oral cavity." Moreover, the non-involved parts of the head and neck were covered by a shield. The radiation was delivered concurrently with intravenous cisplatin administered with either 100 mg/m2 divided in 3 days every 3 weeks or 35 mg/m2 weekly doses. The intervention group received celecoxib 100mg qid (7 days/week), while the control group received placebo from the initiation of chemoradiation to one week after the last fraction of radiotherapy for 8 weeks. Our study protocol mandated a minimum of 5 weeks of celecoxib consumption.
3.4. Toxicities
The patients were evaluated weekly for acute side effects such as mucositis. Trained physicians who were blinded to the study group assignment assessed the side effects based on the 3rd version of Common Terminology Criteria for Adverse Events (CTCAE). The patients received their drugs for the incoming week in each visit. The patients were asked to deliver the remained capsules of the previous week to assess their compliance.
Standard oral care protocols were permitted. The authors insisted on the consistency in the use of institutional protocol (diphenhydramine, lidocaine, aluminum hydroxide) at the beginning of grade 2 toxicities. The patients received instruction on oral care at the beginning of treatment and were encouraged to care about oral health if deemed appropriate. Commercial mouthwashes (benzydamine and chlorhexidine) were prohibited. Blood profile (hemoglobin level, white cells, and platelets count), blood urea nitrogen (BUN) and creatinine (Cr), and body weight were checked and recorded weekly. Main side effects attributable to celecoxib including gastrointestinal toxicities were assessed in regular visits during treatment.
The follow-up of each patient was done periodically after the end of chemoradiation every 3 months for 2 years and every 6 months after that. Each visit included history and physical examination and computed tomography (CT) scan or magnetic resonance imaging (MRI) as indicated. Primary visits up to 12 months following the completion of treatment included lab tests to assess renal function.
3.5. Clinical Response and Oncologic Outcomes
Clinical response evaluation was made 3 months after the end of chemoradiation. We defined complete response as “no evidence of disease in the primary location or neck based on physical examination and imaging.” The local-regional control was defined as the absence of recurrence in the primary location or the neck among the complete clinical responders. The overall survival rate was calculated from randomization to the last follow-up or death due to any reason. The progression-free survival rate was derived from the time of randomization to the time of treatment failure (local-regional recurrence or distant metastases), death, or the last uneventful follow-up visit.
3.6. Statistical Methods
The primary endpoint was the reduction in acute side effects, while the secondary endpoints included response rate, loco-regional control, progression-free survival (PFS), distant metastasis-free survival (DMFS), and overall survival (OS).
The study power was 80%, the significance level was 0.05, and the drop-out rate was 10% in the formula to calculate the sample size. The final size of each group was 60 subjects.
We applied the Kaplan-Meier method (including life tables) to perform time-event analysis (OS and PFS rate, and local-regional control rate) in an intention-to-treat fashion. Also, Cox proportional hazards and log-ranked tests were utilized to compare variables between study groups. To observe and analyze the trend of toxicities, during the treatment, the authors utilized the analysis of variance (ANOVA) for repeated measures. To compare the response rates between groups at 3-months post-treatment, we used a chi-squared test. All the crucial rates were presented with a confidence interval of 95% (CI95%) for the measure. Finally, to obscure the effect of potential confounders, we used multivariate regression analysis so that we could see the impact of celecoxib versus placebo independently.
4. Results
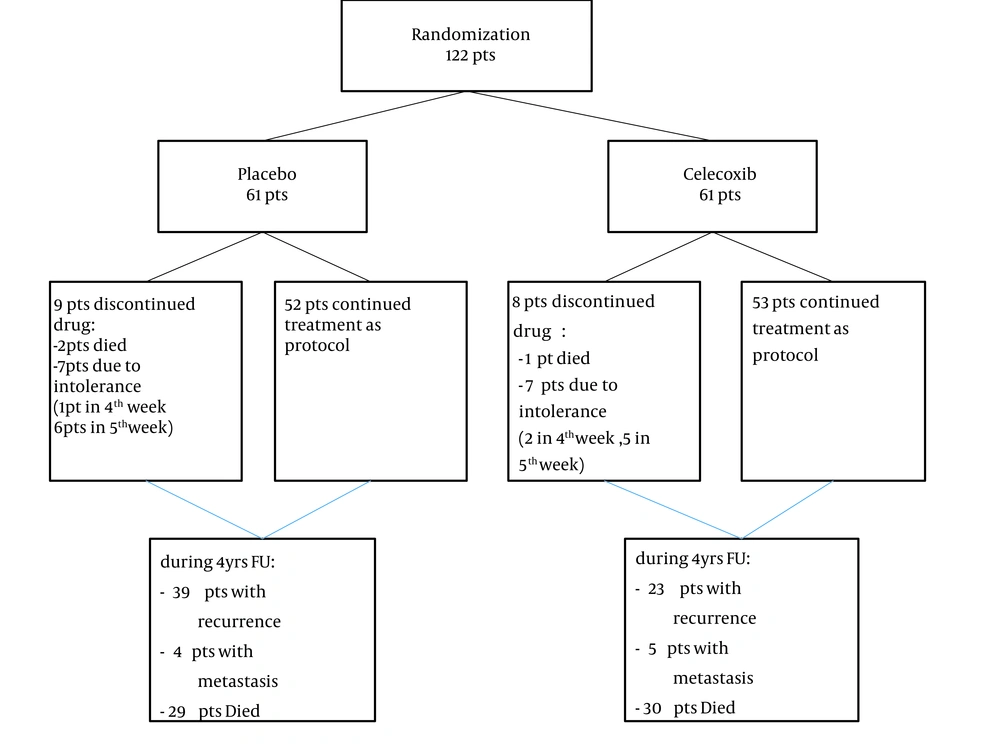
Totally, 122 patients were enrolled in the study (61 patients in each group) and were followed up 3 to 50 months (median follow-up time: 30 months). No statistically significant difference was noted between the treatment groups in any of the subject or disease characteristics (Table 1). Seventeen patients (14%) did not complete the treatment protocol (receiving the COX-2 inhibitor or placebo for at least five weeks after the onset of radiotherapy) because of drug intolerance or death during the first 5 weeks of treatment. The study diagram is depicted in Figure 1.
| Parameter | COX2 (N = 61) (%) | Placebo (N = 61) (%) | P-Value |
|---|---|---|---|
| Age (mean ± SD, y) | 56.46 ± 14.65 | 55.08 ± 12.45 | 0.867 |
| Gender | 0.848 | ||
| Male | 40 (65.6) | 41 (67.2) | |
| Female | 21 (34.4) | 20 (32.8) | |
| Primary tumor site | 0.539 | ||
| Oral cavity | 14 (23) | 12 (19.7) | |
| Oropharynx | 2 (3.3) | 4 (6.6) | |
| Larynx | 12 (19.7) | 19 (31.1) | |
| Hypopharynx | 8 (13.1) | 4 (6.6) | |
| Nasopharynx | 19 (31.1) | 17 (27.9) | |
| Others | 6 (9.8) | 5 (8.2) | |
| T- Stage | 0.870 | ||
| T1 | 2 (3.3) | 3 (4.9) | |
| T2 | 12 (19.7) | 14 (23) | |
| T3 | 26 (42.6) | 22 (36.1) | |
| T4 | 21 (34.4) | 22 (36.1) | |
| N- Stage | 0.820 | ||
| N0 | 21 (34.4) | 17 (27.9) | |
| N1 | 19 (31.1) | 20 (32.8) | |
| N2 | 13 (21.3) | 13 (21.3) | |
| N3 | 8 (13.1) | 11 (18) | |
| Tumor grade | 0.902 | ||
| Well-differentiated | 12 (19.7) | 10 (16.4) | |
| Moderately | 5 (8.2) | 6 (9.8) | |
| Poorly | 6 (9.8) | 8 (13.1) | |
| Undifferentiated | 38 (62.3) | 37 (60.7) | |
| Duration of radiotherapy (mea ± SD, days) | 49.81 ± 16.07 | 46.16 ± 10.56 | 0.156 |
| Radiation dose (mean ± SD, cGy) | 66 ± 4.4 | 66 ± 4.4 | 0.693 |
| Number of fraction of radiation (meanSD, cGy) | 33 ± 2.2 | 33 ± 2.2 | 0.430 |
| Chemotherapy protocol | 0.999 | ||
| Weekly | 26 (42.6) | 26 (42.6) | |
| Every three week | 35 (57.4) | 35 (57.4) |
Patients’ and Disease Characteristics
4.1. Time to Develop Objective Mucositis
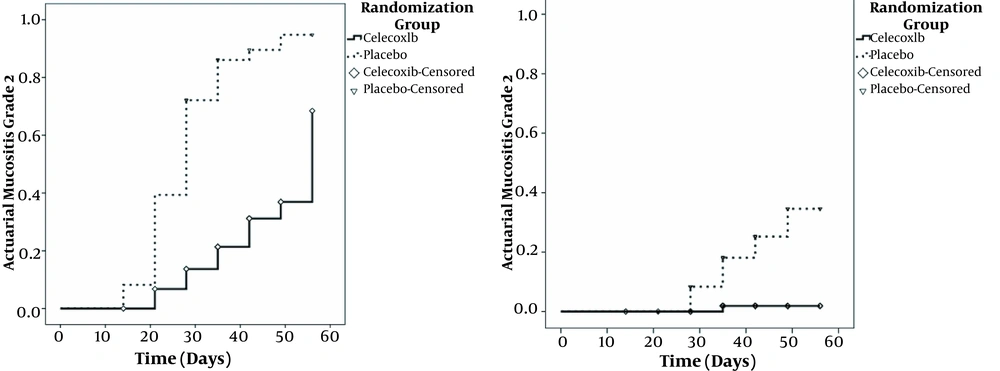
Kaplan-Meier survival analysis on time to develop grade 2 mucositis showed a significant difference between the two groups; in the celecoxib group, it was 56 days [confidence interval (CI)95%: 49 - 63] vs. 28 days (CI95%: 26 - 30) in the placebo group, P < 0.001.
Despite applying the mouthwashes and analgesics in both arms at the beginning of the grade 2 mucositis, there was a significant difference in the rate of grade 3 mucositis between the two groups. Grade 3 mucositis was found just in one patient (1.6%) in the COX-2 inhibitor group compared with 13 patients (21.3%) in the placebo group (P = 0.001). Figure 2 shows the percent of patients with objective grades 2 and 3 in two groups during radiotherapy.
4.2. Development of Oral Mucositis During 5 Weeks of Treatment
ANOVA was performed to determine if the mucositis score changed significantly during the first 5 weeks. As mentioned above, 17 patients (14%) did not continue the treatment for the whole of 5 weeks; so, they were excluded from this analysis. The analysis showed a significant time-by-group interaction (F = 30.226, P < 0.000), time effect (F = 204.26, P < 0.000), and group effect (F = 73.99, P < 0.000). The mucositis and dysphagia scores increased over time in both groups, but the increase was more pronounced in the placebo group than in the celecoxib group.
4.3. Changes in the Body Weight and Laboratory Parameters
Laboratory tests, including white blood cells (WBC), hemoglobin, and platelet count decreased over 5 weeks in both treatment and placebo groups. However, these declining patterns were not statistically different between the two arms. Based on ANOVA for repeated measures, the weight of subjects significantly decreased within both study groups from the 1st to 6th week. The observed decrement in weight measurements, although it was more significant in the placebo group but did not differ statistically significant between groups (F [2.34, 133.67] = 2.56, P = 0.072).
4.4. Celecoxib-related Side Effects
In our study, we did not encounter any significant side effects attributable to celecoxib.
4.5. Response Rate
The median follow-up duration in the present study was 30 months (range: 3-50). Forty-eight patients (78.6%) in the celecoxib group had complete responses vs. 29 (47.5%) in the placebo group. For the partial response, these figures were 8 (13.1%) vs. 15 (24.5%), and for non-responsive/progressive disease, there were 4 (6.6%) vs. 15 (24.5%) in the celecoxib and placebo groups, respectively (P = 0.04).
4.6. Overall Survival, Local-Regional Control, Progression-free, and Metastasis-free Survival
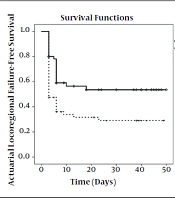
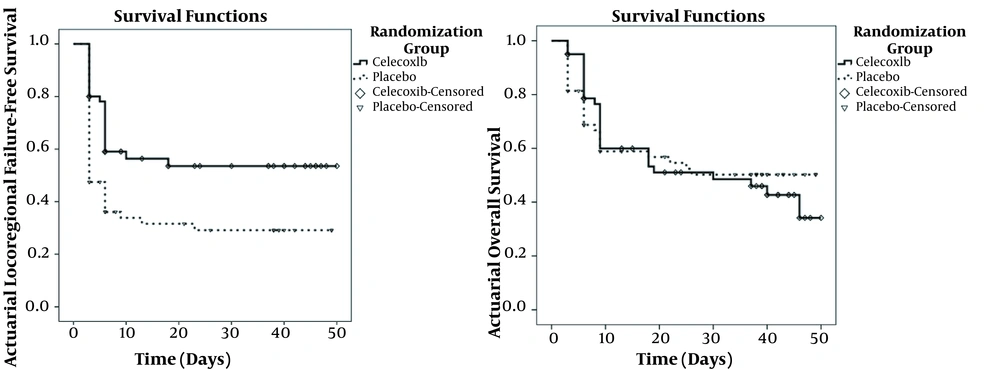
Kaplan-Meier survival analysis was utilized to compare the treatment outcomes in two groups. Three patients died during radiotherapy in the placebo and celecoxib groups due to their basic illness. Thirty (49.2%) and 29 (47.5%) patients of the celecoxib and placebo groups died during the follow-up, respectively. The median overall survival time was 40 months (95%CI: 37.6 - 42.3) and 38 months (95%CI: 36.8 - 39.1) in the celecoxib and placebo groups, respectively (Figure 3).
Twenty-three patients (37.7%) in the celecoxib group and 39 patients (63.9%) in the placebo group experienced loco-regional recurrences/progression during the follow-up. The 4-year local-regional control rates were 51% (CI95% = 37.3 - 64.7) and 28% (CI95%: 16.3 - 39.7) in the celecoxib and placebo groups, respectively. Local-regional failure-free survival is depicted in Figure 3.
Five patients (8.19%) in the Celecoxib group and 4 patients (6.56%) in the placebo group had distant metastasis during the follow-up. Median metastasis-free survival time was 45 months (95%CI: 41 - 49) in the celecoxib group and 46 months (95%CI: 42 - 49) in the placebo group. The 4-year metastasis-free survival was 86% vs. 91%.
5. Discussion
Oropharyngeal mucositis is common among patients undergoing chemoradiation for head and neck cancers. In some patients, symptomatic mucositis leads to treatment interruption, which may adversely affect the response rate and reduce local control and survival. Mucositis also causes a significant economic burden due to necessity hospitalizations, during which utilization of parenteral nutrition and narcotics might be necessary.
Pathophysiologic concepts have shown that oral mucositis has an initial inflammatory/vascular phase, then, an epithelial phase, a (pseudomembranous) ulcerative/bacteriological phase, and finally, a healing phase (8). Radiation therapy and chemotherapeutics are both potent activators of NF-kB. It has been shown that NF-kB would upregulate COX-2. COX-2 has a crucial role in the production of prostaglandins from arachidonic acid, and it has been found that COX-2 was an amplifier of the toxicity of the mucosal injury and exacerbation of severity and duration of mucositis (9).
The first report of a role for COX inhibitor in the radiation-induced mucositis was from a non-selective COX inhibitor, indomethacin, in reducing the severity of mucositis and esophagitis that showed significantly delayed mucositis onset (10). Another study that has evaluated the effect of celecoxib on acute side effects of radiotherapy in head and neck carcinoma was a phase 1 trial in nasopharyngeal carcinoma that showed a likely protective impact and increased response rate (11).
COX-2 is not only important in inflammation, but it has a crucial role in tumor angiogenesis by the means of vascular endothelial growth factor (VEGF) activation, decreasing the rate of tumor cell apoptosis, and enhancing tumor radio-sensitivity (12). COX-2 and its products may have a stimulatory effect on tumor growth and metastases. Increased levels of COX-2 have been proved in a variety of human malignancies including head and neck cancer, in which 100% of the cancerous squamous cells overexpress COX-2 (5). In several carcinomas, COX-2 overexpression correlates with aggressive behavior, poor prognosis, and the development of metastatic disease (13).
Phase-I, II, and III clinical trials for the combination of celecoxib with chemotherapy and radiotherapy were conducted in various solid malignancies. A combination of a COX-2 inhibitor and chemotherapy showed promising results in lung, colorectal, esophageal, pancreatic, breast, and brain cancers with improving the efficacy of chemotherapy drugs by increasing response rate and decreasing acute toxicities (14-19). Nevertheless, the results of limited published phase III trials were contradictory so that some are against the combination of celecoxib and standard treatment. The results of CYCLUS and NVALT-4 studies (19, 20) for advanced non-small cell lung cancer showed that the addition of celecoxib to chemotherapy did not improve survival and COX-2 overexpression was not a prognostic biomarker and had no predictive value. In colorectal cancer combination of celecoxib with chemoradiation improved response rate and down-staging from 35% to 61%, although not significant (21). Celecoxib, in conjunction with the FOLFOX-4 regimen in advanced colorectal cancer, showed that the 3-year survival rate was significantly better in the celecoxib group (22). It was interesting that in both trials, the anti-tumor and chemo-sensitizing effects of celecoxib appeared to be independent of COX-2 overexpression.
A phase III trial by Mohammadianpanah et al. on head and neck cancers (23), which had combined administration of celecoxib with concurrent weekly cisplatin-chemoradiation in nasopharyngeal carcinoma, showed that addition of celecoxib (100mg bid) to concurrent chemoradiation and adjuvant chemotherapy after that was associated with improved 2-year local-regional control rate from 84% to 100%. However, overall survival was not significantly different (88% vs. 84%). The acute side effects, such as xerostomia, mucositis, and myelosuppression were similar. Moreover, a recent double-blind, randomized placebo-controlled study reported no clinical significance in reducing mucositis and oral pain following the utilization of celecoxib (24).
Our patients on celecoxib had a better toxicity profile and better progression-free survival than the placebo group. However, in longer follow-up, celecoxib did not improve overall survival. The almost same OS in both groups of the locally advanced head and neck carcinomas could be the result of applying extensive surgical procedures for salvage treatment of refractory or relapsed diseases (the hospital protocol at the time of investigation). Therefore, the OS of patients with an unfavorable PFS or LRC could be improved, employing surgical interventions.
COX-2 inhibitors have advantages not only for enhancing tumor response to radiotherapy and chemotherapy but also for their protection against treatment-related gastrointestinal complications. Although the COX-2 inhibitors have not shown an impact on the overall treatment time, they led to a better tumor local control and limited the GI side effects of the radiation therapy. These therapeutic achievements could be translated into a higher quality of life for the patients. However, there are some concerns regarding COX-2 inhibitors having pro-thrombotic features and increasing the risk of myocardial infarction. For instance, the adenoma prevention with celecoxib (APC) trial demonstrated a markedly higher risk of myocardial infarction in patients receiving celecoxib vs. placebo (25). The majority of celecoxib trials were with 800 mg daily and for a long duration, but in our study, patients have been on a lower daily dose (400 mg) and a maximum period of 8 weeks. A review of 6 randomized trials of cardiovascular risk of celecoxib showed that the hazard ratio for all dose regimens was associated with baseline cardiovascular risks. This hazard ratio was lowest in 400mg daily vs. 200mg bid or 400 mg bid (26); although we did not assess the cause-specific survival, we think that 400 mg daily is a safe dose.
In our study, we had some limitations; considering the single-center nature of this trial, we recruited a limited number of patients with different sites (e.g., nasopharyngeal, laryngeal, hypopharyngeal, oral cavity, and even paranasal sinus carcinomas) in this study, and this non-uniformity (although well balanced between two groups) may affect results of our research on complications and survival. However, the toxicity scores were assessed by the trained physicians, and the patients’ reported subjective outcomes were not included. Furthermore, the nasopharyngeal cancer patients were not excluded due to the limited number of definitive radiotherapy cases in a single-centered population. Another limitation was our failure to acquire information about the need to insert and keep feeding tubes or consume narcotics. Besides, lacking developed radiotherapy methods such as IMRT and 3DCRT and newer linear accelerators at the study time was an important issue that may solely affect the final therapeutic results.
Therefore, we suggest further randomized studies in the more uniform and larger quantity of patients or newer chemoradiation protocols and techniques for a better definition of the role of COX-2 inhibitor in head and neck carcinoma.
5.1. Conclusions
Our RCT study showed that a COX-2 inhibitor (celecoxib) in combination with definitive chemoradiation provides a significant therapeutic gain in head and neck cancer. Recent advances in radiation therapy techniques like IMRT have lowered treatment-related side-effects or improved response. The results of this study could best suit cancer centers with limitations to consume the newest technologies.