1. Background
Laparoscopic sleeve gastrectomy (LSG) is considered one of the most common surgical procedures for morbid obesity (1). LSG is a safe bariatric procedure; it has recently acquired its place in bariatric surgery as a preliminary procedure. In the literature, the estimated frequency of incidental pathology during laparoscopic bariatric surgery was approximately 2%. Among these, gastrointestinal stromal tumors (GISTs) are an infrequent finding during LSG, with an incidence of lower than 1% among all bariatric procedures. Resection with negative microscopic margins (R0) through partial gastrectomies is the most appropriate treatment (2).
GISTs are most commonly located in the stomach mainly the fundus area (3). Most of the GISTs are of spindle cell tumors (70% of patients) and 99% due to the expression of CD117 (c-kit protein). GISTs are the commonest mesenchymal tumors with both mural and neural origin (4, 5).
2. Objectives
The incidence of GISTs in patients, who underwent LSG and resection with a 1- to 2-cm safety margin, were validated and analyzed. The primary endpoint is that can simultaneous excision be oncologically adequate or not? How much GIST is supposed to be far from a staple line?
3. Methods
3.1. Study Population
The current double-center prospective study included 338 morbidly obese patients with age ≥ 20 years and body mass index (BMI) ≥ 35; 17 patients in Zagazig University Hospitals, Faculty of Medicine, Egypt, and 321 patients in the bariatric surgery excellence unit in a tertiary hospital in Riyadh, KSA. All patients admitted for LSG without any known history or imaging reveal GIST, which was detected intraoperatively during LSG and diagnosis settled by histopathological examination.
3.2. Ethical Approval and Clinical Registration
The present study was approved by the Institutional Review Board of Faculty of Medicine, Zagazig University Hospitals under the code IR-261102-1. Informed consent was obtained from all participants. The present research was registered in ClinicalTrial.gove with a unique protocol ID: NCT04344847.
3.3. Protocol and Setting
The inclusion criteria included age ≥ 20 years and all morbidly obese patients had body mass index (BMI) ≥ 35.
The exclusion criteria included previous gastric surgery, symptomatic reflux, and hiatus hernia.
This work has been reported in line with strengthening the reporting of cohort studies criteria (STROCSS) (6).
3.4. Pre-Interventional Protocol
Basic and clinical characteristics were defined, including gender, age, BMI, comorbidities, tumor characteristics (localization, number, tumor size), and histopathological criteria, mainly mitotic count and immunohistochemistry for markers.
All patients underwent a preoperative workup panel that was recorded in our database including blood picture, coagulation profile, liver and kidney function, thyroid function, neck and abdominal ultrasound, chest x-ray, and upper gastrointestinal endoscopy (UGIT).
3.5. Intervention
3.5.1. Procedure
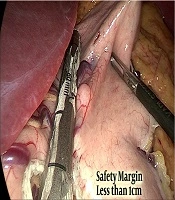
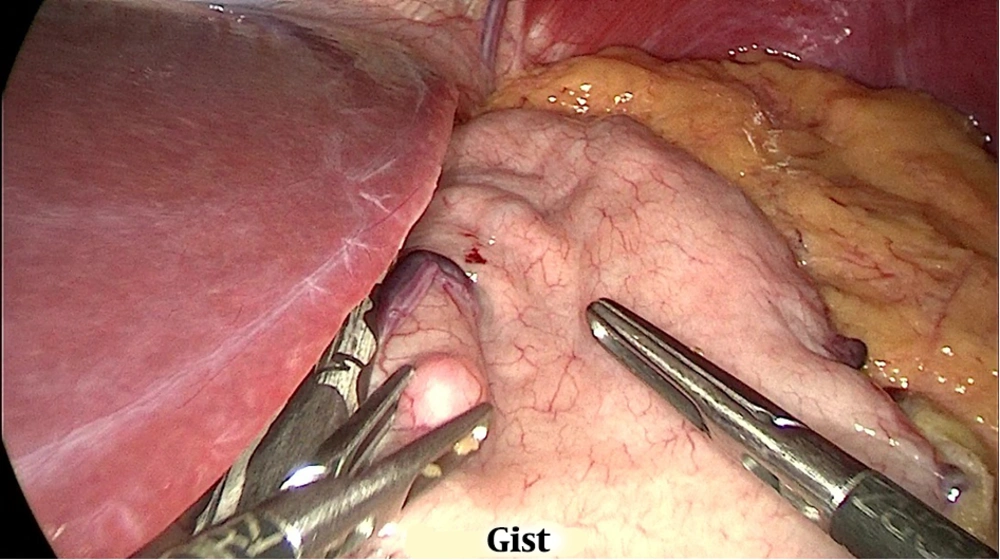
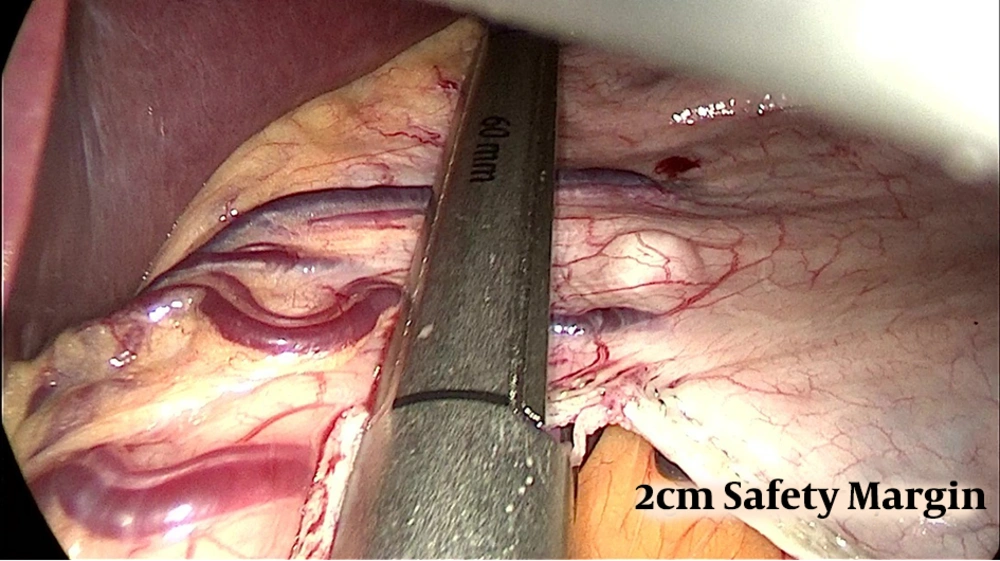
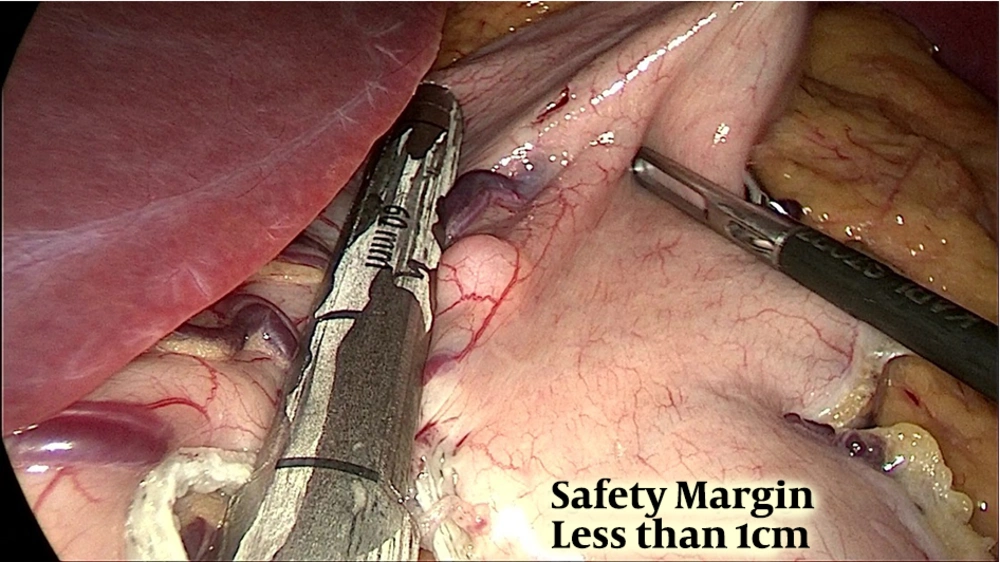
Preoperative assessment in the anesthesia clinic was done. The procedure was performed under general anesthesia. Pneumoperitoneum was done with pressure of 15 mmHg and 17 mmHg for some patients via visiport in the left Mid-clavicular line 2 fingerbreadth below the costal margin. Standard placement of 3 more trocars was done. The second port supraumbilical to the left directly looking to the pylorus, the third supraumbilical to the right of the umbilicus, and the last 5mm port at epigastrium. The surgeon stood between the patient’s leg, using the first and third port while a camera through the second port. The abdominal cavity was inspected to rule out any other pathology. Dissection devascularization of the greater curvature of the stomach started approximately 6 cm proximal to the pylorus and the stomach was sleeved over a 36 Fr calibration tube. A thorough examination of the whole stomach with specific attention to the posterior surface to release any adhesions was mandatory before the initiation of resection. An incidental polypoidal lesion was found (Figure 1) in 17 patients. Most lesion located in the fundus or the mid-portion of the stomach was segmented and resected, aiming at achieving a good safety margin (wide margin) 1 - 2 cm (Figure 2), but some cases only marginal margin (1 cm or less) (Figure 3) to avoid incorporation in staple line. Staple line integrity was reinforced with staples (Figure 4). The stomach was retrieved in Endo-bag. The resected specimen was examined and the margin was measured, using a ruler; the GIST polypoidal lesion was defined 1 - 2 cm away from the stapled line and, then, sent for histopathology and immunohistochemistry. The staging was done according to the latest edition of AJCC (8th edition), TNM Staging, where tumor size was estimated from the histopathological reports for T1 less than 2 cm and T2 more than 2 cm. In our procedure, margins were defined as wide margin ≥ 2 cm and marginal margins ≤ 2 cm.
3.6. Post-Intervention
The Multi-Disciplinary Tumor Board Committee approved to report the data based on the American Joint Commission on Cancer and the Union for International Cancer Control (AJCC/UICC) TNM, 8th edition.
LSG was run smoothly. On the next day, the gastrografin swallow meal showed the good passage of contrast from the oesophagus into the stomach and onwards into the duodenum. No evidence of any leakage was noted.
3.7. Post-Operatively
Our patient tolerated the procedure without intra-operative major complications and had a smooth and uneventful hospital course. On the day of surgery, patients kept nothing by mouth, on postoperative day one, started 30 mL water every 30 minutes till postoperative day 2. Thereafter, patients began on clear oral liquids.
3.8. Follow-Up
Patients’ vital signs were monitored on the day of surgery regularly every 4 hours, early mobilization was required, and the recommended thromboprophylaxis regimen was received. Patients were discharged 2 or 3 days later.
Follow-up was done regularly; the first visit was after 10 days for stitch removal, the prescription of vitamin supplementation, and following the results of histopathology and immunohistochemistry. All patients underwent a computed tomography (CT) follow-up at 3 - 6 months and upper GIT endoscopy at 12 and 24 months follow-up.
3.9. Statistical Analysis
Patients' data were documented, gathered, and analyzed, using SPSS 22.0 for windows and Microsoft Office Excel 2010. Continuous quantitative variables were expressed as the mean ± SD and median (range), and categorical qualitative variables were expressed as absolute frequencies (number) & relative frequencies (percentage).
4. Results
From November 2016 to May 2018, 338 patients underwent LSG at 2 institutions. Seventeen patients were detected with unexpected coincidental GISTs during the LSG, resulting in an incidence of 5%.
A total of 14 females with GISTs (84%) and 3 males with GISTs (16%) constituted the patients. The age of patients with discovered GIST ranged from 21 to 41 years with the mean ± SD of 31.91 ± 5.70 and a median of 31 (21 - 41). The median preoperative weight range was 134 (112 - 170) kg with the mean ± SD preoperative BMI of 41.16 ± 6.35 kg/m2.
All basic characteristics of operated patients (n = 338) are listed in Table 1.
| Patients’ Characteristics | The Operated Patients (N = 338). No. (%) |
|---|---|
| Sex | |
| Male | 254 (75.1) |
| Female | 84 (24.9) |
| Age, y | |
| Mean ± SD | 31.91 ± 5.70 |
| Median (range) | 32 (21 - 41) |
| Comorbidities | |
| Absent | 139 (41.1) |
| Present | 199 (58.9) |
| Type II DM | 122 (36.1) |
| Hypertension | 124 (36.7) |
| Dyslipidemia | 123 (36.4) |
| History of breast cancer | 1 (0.3) |
| History of ovarian cancer | 1 (0.3) |
| Preoperative weight, kg | |
| Mean ± SD | 137.39 ± 20.01 |
| Median (range) | 134 (112 – 170) |
| Preoperative BMI, kg/m2 | |
| Mean ± SD | 41.16 ± 6.35 |
| Median (range) | 37 (35 - 50) |
| Preoperative dyspepsia | |
| Absent | 100 (29.6) |
| Present | 238 (70.4) |
| Preoperative chronic gastritis | |
| Absent | 258 (76.3) |
| Present | 80 (23.7) |
| Preoperative Helicobacter pylori | |
| Absent | 278 (82.2) |
| Present | 60 (17.8) |
| Operative time, min | |
| Mean ± SD | 49.45 ± 4.16 |
| Median (range) | 50 (5 - 57) |
| Incidental GIST | |
| Absent | 321 (95) |
| Present | 17 (5) |
Basic Characteristics of Operated Patients (N = 338)
When analyzing the presence of comorbidities in GIST patients, 36.7% (n = 124) had a history of hypertension, 8 patients had GISTs with type II diabetes mellitus (DM), and 36.1% (n = 122) were involved with dyslipidemia, from which 8 had GISTs. Only one female patient reported having a history of ovarian and breast cancer. At the time of examination and admission, she was disease-free.
Generally, 17% of patients (n = 12) reported dyspepsia as the main complaint in GIST patients. None of the patients had symptoms, laboratory tests, or imaging that helped to settle a preoperative diagnosis of GIST.
All patients underwent preoperative UGIT as a part of the preoperative workup. The findings were non-suggestive of GIST in any case; 18 patients were excluded due to a hiatus hernia. Superficial chronic gastritis and gastroduodenitis were some of the most common findings encountered in the endoscopic biopsy. Only 5 patients with GIST patients (30%) showed that gastritis; 19% of GIST patients were Helicobacter pylori-positive, which was treated before surgery by triple therapy. But many cases recorded H. pylori positive postoperatively.
The mean ± SD of operative time was 49.45 ± 4.16 minutes and the median (range) was 50 (41 - 57) minutes. No change in the already planned procedure was necessary upon coincidental detection of GISTs.
The Clinico-pathological data of discovered incidental GIST (n = 17) were illustrated in Table 2.
| Clinico-Pathological Data | Incidental GIST (N = 17), No. (%) |
|---|---|
| Location of tumors | |
| Fundus and cardia | 15 (88.2) |
| Body | 2 (11.8) |
| Tumor extension | |
| Intraluminal | 2 (11.8) |
| Extraluminal | 12 (70.6) |
| Transluminal | 3 (17.6) |
| Number of tumors | |
| Single | 17 (100) |
| Tumor size, cm | |
| Mean ± SD | 1.41 ± 0.40 |
| Median (range) | 1.50 (0.60 - 2.10) |
| Mitotic index | |
| Low rate | 16 (94.1) |
| High rate | 1 (5.9) |
| CD 117 | |
| Negative | 1 (5.9) |
| Positive | 16 (94.1) |
| DOG1 | |
| Negative | 2 (11.8) |
| Positive | 15 (88.2) |
| SMA | |
| Negative | 17 (100) |
| S100 | |
| Negative | 1 (5.9) |
| Positive | 16 (94.1) |
| Tumor rupture | |
| Negative | 16 (94.1) |
| Positive | 1 (5.9) |
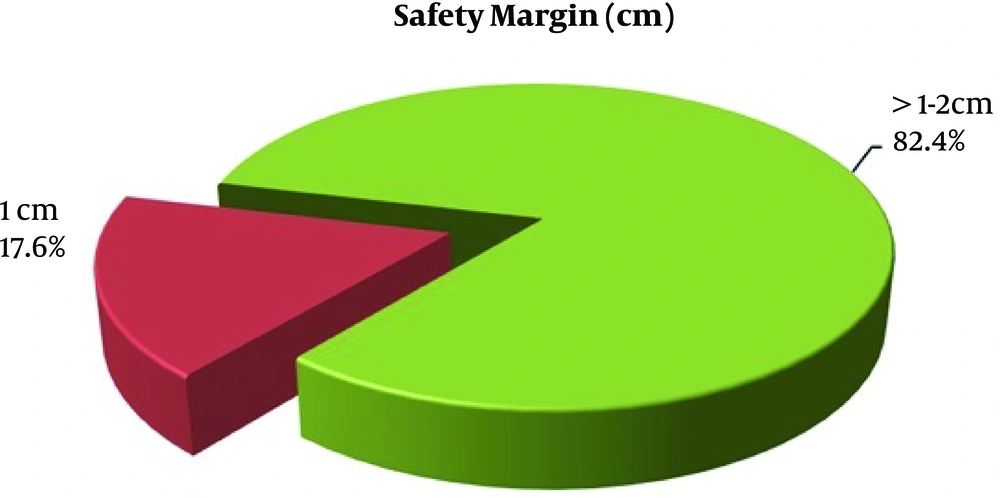
| Safety margin, cm | |
| Marginal margin ≤ 1 | 3 (17.6) |
| Wide margin > 1 - 2 | 14 (82.4) |
| Surgical margin | |
| Negative | 15 (88.2) |
| Positive | 2 (11.8) |
| Regional lymph nodes | |
| Not revealed | 17 (100) |
| T | |
| T1 | 15 (88.2) |
| T2 | 2 (11.8) |
| AJCC TNM | |
| Stage IA | 16 (94.4) |
| Stage IIA | 1 (5.9) |
| Recurrence | |
| Absent | 17 (100) |
Clinico-Pathological Data of Discovered Incidental GIST (N = 17)
Location of the Tumor: Most of the GISTs were found along the greater curvature, mainly in the fundus in 15 patients (88.2%) and only 2 patients with GISTs in the body.
Histopathological examination was performed with an Immunohistochemically essay. The tumor was of spindle cell in 88%. Mitotic rate was calculated and 16 patients (94.1%) had a low rate of 0 - 4/5 mm2 high power field or fewer mitoses per 5 mm2 and 1 patient (5.9%) had more than 5 mitoses per 5 mm2.
Tumor size ranged from 0.5 cm T1 to 2.1 cm T2 with majority T1; 15 patient (88.2%) and 2 patients fall in T2 (11.8%). The staging was done according to TNM; stage IA: 16 patients and stage IIA: 1 patient.
Growth pattern and tumor extension: 12 patients (70.6%) have an extraluminal tumor, 3 transluminal, and 2 intraluminal.
Tumor Rupture: Only 1 patient was found with tumor rupture, who was a positive margin status.
Immunohistochemically: 94.1% were positive for CD117, 88.2% were positive for DOG1, 94.1 % were positive for S100 protein, and 100% were negative for SMA.
Safety margin (Figure 5) was marginal margin ≤ 2 cm for 3 patients to avoid incorporation in staple line and wide margin in 14 patients (82.3%) ≥ 2 cm. Resection margin status was assessed in biopsy and revealed positive margin R1 for 2 patients, in which their safety margin was only 1 cm or less and one of them had high mitotic rate staged as IIA.
Margin status revealed 15 patients with negative margin (R0), 2 patients (11.8%) with positive margin (R1), 1 of them underwent re-exploration, where total gastrectomy and esophagojejunostomy was performed; the other patient refused intervention and received Imatinib. The patient received treatment (400 mg/day) 1 year after the patients were informed about the effects, duration of Imatinib, and prognosis. All patients underwent a CT follow-up at 3 - 6 months, upper GIT endoscopy 12 and 24 months follow-up; chronic superficial gastritis was one of the most common histological findings in 93% of cases; 82% of them showed atrophic type even in the patient receiving Imatinib. CT abdomen and pelvis with oral and intravenous contrast was performed in all patients, showing no evidence of metastasis even in the patient who received Imatinib.
5. Discussion
GIST are rare mesenchymal tumors, most commonly arising in the stomach. They account for less than 1% of gastrointestinal tumors, constituting the most common mesenchymal neoplasm of the gastrointestinal tract (2).
In this prospective multi-center study, we evaluated the feasibility of GIST resection during LSG and calculated the incidence of coincidental GISTs encountered during LSG for morbidly obese patients to be 5%. The incidence in our study is higher than reported in the literature, which is 0.6% - 0.8%. Other authors reported a different incidence, Yuval et al. (7) reported an incidence near that in literature, which is 0.6% among 827 patients who underwent LSG. Chiappetta et al. (8) studied 2603 patients and reported an incidence of 0.31% (3 per 1000). Viscido et al. (2) reported 5 (0.5%) patients were found to have incidental GIST.
A total of 14 females with GISTs (84%) and 3 males with GISTs (16%) constituted the patients. The age of patients with discovered GIST ranged from 21 to 41 years with the mean ± SD of 31.91 ± 5.70 and the median of 31 (21 - 41). The median preoperative weight range was 134 (112 - 170) kg with the mean± SD preoperative BMI of 41.16 ± 6.35 kg/m2. No significant difference related to demographic data impacted the coincidental discovery of GIST. Lyros et al. (9) reported an increasing incidence of coincidental GIST in 9 (1.27%) patients. Seven (78%) incidental GIST tumors were detected in the females with a mean age of 55.6 years and ranged from 27 - 74 years; the mean BMI ranged 38 - 71 mg/m2.
All unexpected GISTs were detected intra-operatively and resected simultaneously with the same specimen of LSG in all patients. All patients were asymptomatic for GIST preoperatively and whatever size of GIST encountered during LSG; no preoperative symptoms were encountered. Zhao and Yue (10) noted that up to 75% of GISTs are discovered when they are less than 4 cm in diameter and nearly one-third of GISTs discovered in his cases were asymptomatic, and symptomatic cases had vague non-specific symptoms.
The preoperative workup of upper GIT endoscopy may detect abnormal findings, detect tumors larger than 2 cm but mostly smaller lesions may be skipped (11). This is compatible with the Chiappetta et al. (8), who declared that a wide range of abnormal endoscopic findings in morbidly obese patients, for that, preoperative endoscopy should be considered for all patients undergoing LSG. In our study, all tumors were located near the staple line on the serosal side. We found positive helicobacter gastritis in 17.8% of all patients with and without GIST undergoing upper GIT endoscopy; this was compatible with the previous conclusions in the literature with no association between positive HP and GIST (7).
Immunohistochemically, we found 94.1% positive for CD117-87% from which 88.2% positive for DOG1, 94.1 % positive for S100 protein, 61% for CD34, and negative for SMA. This was compatible with most of the studies in the literature. There is another concept of Zhu et al. (12), who stated that Ghrelin associated with the development of GISTs; some GISTs expressed the ghrelin hormone marker and its relevant receptors. But up till now, no reports in the literature specified or clarified whether ghrelin is involved or not.
Tumor size ranged from 0.5 cm T1 to 2.1 cm T2 with majority T1; 15 patient (88.2%) and 2 patients fall in T2 (11.8%). The staging was done according to TNM; stage IA: 16 patients and stage IIA: 1 patient. Safety margin was marginal margin ≤ 2 cm for 3 patients to avoid incorporation in staple line and wide margin in 14 patients (82.3%) ≥ 2 cm. Resection margin status was assessed in biopsy and revealed positive margin R1 for 2 patients, in which their safety margin was only 1 cm or less and one of them had high mitotic rate staged as IIA. We discovered that margin 1 cm is not adequate as proved by margin status in histopathology. This is compatible with Ahlen et al. (13), who confirmed that, based on his study, wide surgical margins are of significant prognostic importance and defined wide margin that with at least 2 cm far from resection margin.
The coincidental finding of GISTs during LSG did not change the already planned procedure; all 17 patients underwent the same procedure, and this is attributed to the size encountered and distance from the stable line. For that, laparoscopy appears technically feasible and associated with a better outcome than open surgery. Inaba et al. (14) showed in his study the feasibility and safety of laparoscopy for gastric GIST resection.
Intraoperative assessment of size and distance to the staple line is of utmost importance. Tumor size can change the surgical approach. Inaba et al. (14) and Lin et al. (15) recommended laparotomy tending to be used to treat larger tumors.
The management of small-sized coincidental GISTs less than 2 cm is still a matter of controversy in many institutes and societies. The last recommendation of the National Comprehensive Cancer Network (NCCN) is that complete tumor excision is a must for GISTs larger than 2 cm, and for smaller lesions less than 2 cm, endoscopic surveillance is needed (16-18). On the other hand, the Canadian guidelines mandate resection even for small GISTs less than 1 cm and attribute this to avoid the risk of spread or metastasis (17). Follow-up of our patients depended mainly on endoscopic ultrasonoscopy and CT as an important indicator for recurrence. All patients underwent an upper GIT endoscopy at 12 and 24 months follow-up. Endoscopic ultrasonoscopy and CT scan are important for the identification of recurrence. Raghavendra et al. (19) recommended follow-up with an abdominal/pelvic CT with oral and intravenous contrast every 2 to 6 months for 3 to 4 years.
As reported and declared in all our patients, simultaneous resection of any unexpected GISTs is safe and feasible with negative microscopic resection margins (R0) not less than 1 - 2 cm without changing the already designed procedure and strategy. But we need high numbers of a patient to settle results in the literature.
5.1. Conclusions
The incidence of unexpected GIST in LSG specimens in our series was high in comparison to the case reported in the literature. Any incidental GIST can be removed safely during LSG with negative microscopic resection margins R0 ≥ 2 cm safety margin without changing the procedure. A wide surgical margin improves the outcome for GIST patients. Margins less than 1 cm associated with adverse prognostic factors but we are still in need of higher patient numbers to have a clue in the literature. Preoperative endoscopy and examining the whole stomach during LSG is a must. LSG is the definitive treatment without recurrence at 2-year follow-up