1. Background
Pancreatic cancer is one of the deadliest malignancies and the seventh leading cause of cancer-related death worldwide (1) with a mortality rate of 97.7% (2). The 5-year survival rate is estimated to be 10% in all patients and 32% in patients without distant metastasis (3). Currently, surgery followed by adjuvant therapy is the most promising treatment, although surgery can only be performed in 20 to 30% of the patients (3-5) with only 10 to 20% chance of long-term survival (6, 7). Neoadjuvant treatments or postoperative adjuvant therapies are also associated with increased patient survival (8-10). Moreover, the experience of the surgical team and the age of the patients can also influence the mortality rate and patient survival (11, 12). Some pathological factors such as tumor size, lymph node status, surgical margin, and tumor grade are also considered as prognostic factors (8). Discovering prognostic factors that affect survival rate in patients with pancreatic adenocarcinoma can allow the physician to accurately select subsequent treatments and thus increase survival rates. One of the recently considered factors is lymph node ratio (LNR) which is the ratio of metastatic lymph nodes to total lymph nodes examined.
One of the main prognostic factors in determining survival rate is the presence or absence of involved lymph nodes (LNs), which may be affected by the total number of resected LNs. Although the minimum accepted number of total resected LNs is controversial but it depends on the experience and skill of the surgeon, and the precise pathology examination (13-16).
2. Objectives
Most studies consider long-term survival after surgery in the absence of metastatic lymph nodes (13, 14, 17). Although lymph node involvement is a common condition in most patients undergoing surgery (14, 18), an increase in the number of metastatic lymph nodes can be a sign of poor survival (17). Ultimately, LNR evaluation has been suggested for predicting postoperative survival rate (19-23). There is no certainty about the value of this ratio and conflicting results have been reported (15, 17). Therefore, this study aimed at evaluating the value of LNR for predicting a 5-year survival rate in patients with pancreatic cancer undergoing the Whipple procedure.
3. Methods
This cohort study was performed on 96 patients with pancreatic cancer undergoing the Whipple procedure at Imam Reza Hospital, Mashhad, northeast Iran, during 2014 - 2019. Patients with pancreatic malignancy whose cancer could be resected based on clinical and radiological criteria were included. Patients with the unresectable or borderline resectable disease, presence of distant visceral metastasis, a pathological diagnosis other than adenocarcinoma, and history of receiving neoadjuvant treatments including radiotherapy or chemotherapy were excluded. All patients signed a written informed consent form before surgery. The ethics committee of Mashhad University of medical sciences approved the study. A pylorus-preserving Whipple procedure was performed for all patients and lymphadenectomy including LN stations no. 5, 6, 8a, 12b1, 12b2, 12c, 13a, 13b, 14a, 14b, 17a, and 17b.
Demographic and pathological information including age, sex, tumor differentiation, surgical margin, the total number of lymph nodes examined, and number of lymph nodes in patients with metastasis were recorded. The tumor stage was then determined for each patient based on TNM classification. According to the pathological report of the initial biopsy, the tumor differentiation was as follows: (1) differentiated G1, (2) moderately differentiated G2, and (3) poorly differentiated G3. The condition of the marginal radial tumor (positive/negative) was also defined. The LNR was determined based on the ratio of the number of lymph nodes involved (N1) to the total number of lymph nodes examined, and was divided into 3 levels based on survival sensitivity at different levels of the ratio. In this prospective study, patients were followed up by active phone calls. This study was approved by the Ethics Committee of Mashhad University of Medical Sciences.
All analyses in this study were performed using STATA software, version 12 (STATA Corporation, College Station, TX, USA, 2009). Firstly, all data from the observations were described by type. Descriptive statistics such as frequency (percentage), median, mean and standard deviation were used as appropriate. Survival was calculated using the Kaplan Meier method. Then the relationship between each demographic and pathological factor and survival was evaluated. To determine the accepted cut-off ratio of the lymph nodes, the survival chart was plotted for each of the possible cut-off points, and finally, in the multivariate model, the predictive value of the variables was reported by controlling other variables. P < 0.05 was considered as statistically significant.
4. Results
Of the 96 patients eligible for the study, 51 (53.13%) were men. The mean age of patients was 57.1 ± 14.1 (range: 19 - 82) years and 25 (26.04%) patients were over 65 years of age. The overall 1, 3 and 5-year survival rates for all the patients were 71, 53, and 34%, respectively. The median survival rate was 14.11 months, ranging from 18 days to 60 months.
The median total lymph nodes evaluated in the patients was 7 (range: 1 - 27), and no metastatic lymph nodes were found in 57 (59.37%) patients. In 39 patients with metastatic lymph nodes, 1 to 12 (median: 2) lymph nodes were involved. The median LNR was 0.17 in patients with metastatic lymph nodes. Radial tumor margin was negative in 80 (83.33%) patients and the disease stage was 2 or 3 in 52% of the patients. Moreover, tumor differentiation was moderate or poor in 72 (75%) patients (Table 1). The value of each of the independent clinicopathological factors was evaluated to determine its significance as a prognostic factor and survival was compared through the log-rank test (Table 2).
| Variables | Values |
|---|---|
| Sex | |
| Female | 45 (46.88) |
| Male | 51 (53.13) |
| Age (y) | |
| ≤ 65 | 71 (73.95) |
| > 65 | 25 (26.04) |
| Radial margin | |
| R0 | 80 (83.33) |
| R1 | 16 (16.67) |
| Adjuvant chemotherapy | |
| Yes | 57 (59.38) |
| No | 39 (40.63) |
| Tumor differentiation | |
| Well (G1) | 24 (25.56) |
| Moderate (G2) | 66 (68.75) |
| Poor (G3) | 6 (6.25) |
| Tumor stage | |
| T0 | 8 (8.16) |
| T1 | 12 (12.50) |
| T2 | 55 (57.29) |
| T3 | 2 (21.88) |
| Node stage | |
| N0 | 57 (59.37) |
| N1 | 39 (40.62) |
| Disease stage | |
| 0 | 8 (8.33) |
| IA, IB | 38 (39.58) |
| IIA, IIB, III | 50 (52.08) |
| Tumor size (cm), median (min, max) | 3 (1, 11) |
| Total examined lymph nodes, mean (min, max) | 8.11 (1, 27) |
| Metastatic lymph nodes, mean (min, max) | 1.18 (1, 12) |
a Values are expressed as No. (%) unless otherwise indicated.
| Variable (N) | LNR0 | LNR1 | LNR2 | P-Value |
|---|---|---|---|---|
| Tumor size (cm) | 0.75 | |||
| ≤ 2 | 13 | 8 | 3 | |
| > 2 | 39 | 19 | 14 | |
| Gender | 0.660 | |||
| Female | 26 | 10 | 9 | |
| Male | 30 | 14 | 7 | |
| Radial margin | < 0.001 | |||
| R0 | 52 | 20 | 8 | |
| R1 | 4 | 4 | 8 | |
| Age (y) | 0.10 | |||
| ≤ 65 | 44 | 14 | 13 | |
| > 65 | 13 | 10 | 2 | |
| Tumor stage | 0.161 | |||
| 0 | 8 | 0 | 0 | |
| IA, IB | 38 | 0 | 0 | |
| IIA, IIB, III | 10 | 24 | 16 |
Abbreviations: N, number; LNR, lymph node ratio.
There was no association between sex, tumor size, tumor differentiation rate, disease stage, and the total number of metastatic lymph nodes and overall survival rate; although age (P = 0.04), surgical radial margin (P = 0.001), lymph node status (N0, N1) (P = 0.01) and LNR (P = 0.01) were the most important prognostic factors for survival in the patients.
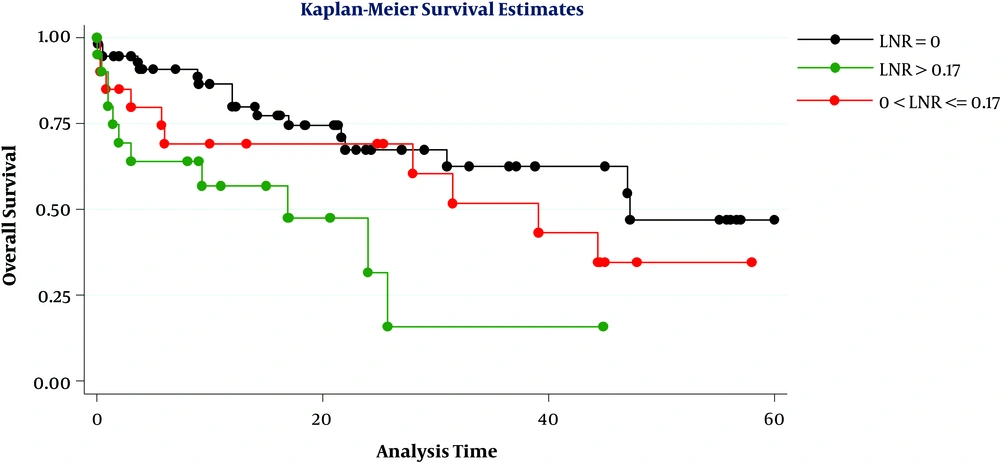
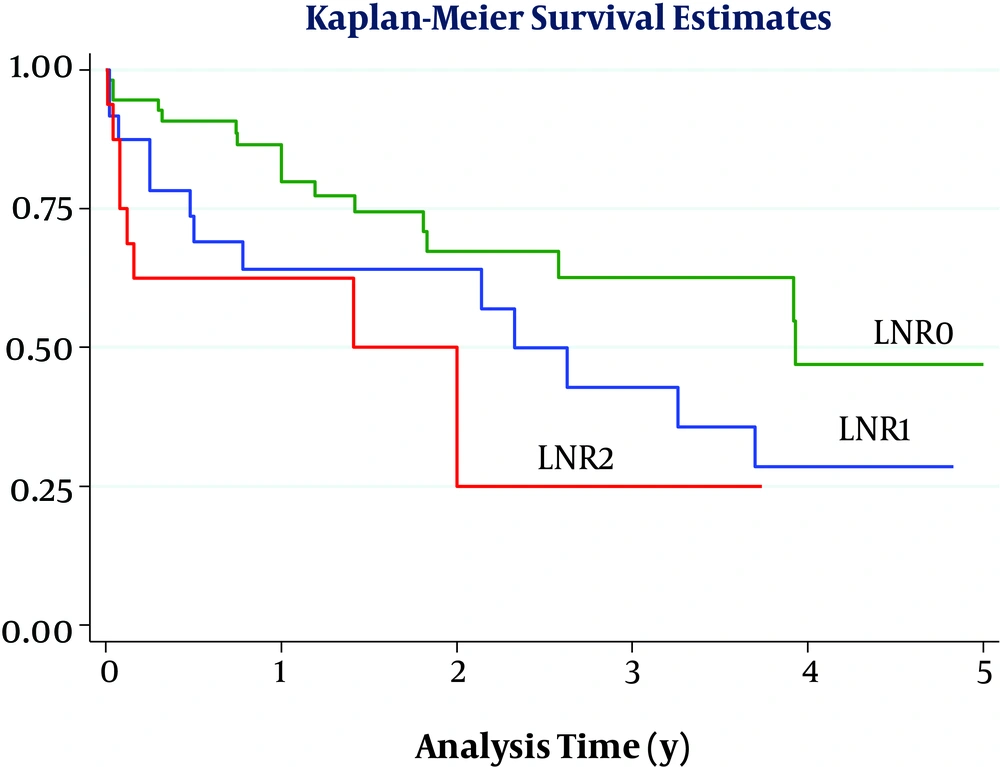
Considering a cut-off point of 0.17, the overall survival of patients was significantly differed when they were divided into 3 groups based on LNR levels (LNR = 0, 0 < LNR ≤ 0.17, and LNR > 0.17) (P = 0.01) (Figure 1). However, this difference was also significant when the cut-off was 0.20 (P = 0.03) (Figure 2). By comparing the figures, we found that the best cut-off for the evaluated data could be 0.20. Ultimately, the results of multivariate analysis showed that in this sample only age of over 65 years was the most important prognostic factor for poor survival in patients (HR = 2.30, CI = 95%, 1.10, 4.78), while LNR more than 0.20 (HR = 2.62, P = 0.05) and positive radial margin (HR = 2.10, P = 0.05) can also predict poor patient survival.
5. Discussion
In this study, which lasted 5 years, 96 patients with pancreatic cancer who underwent the Whipple procedure were followed up to identify prognostic factors affecting their survival rate. Lymph node involvement and positive margin were prognostic factors for poor survival in patients with pancreatic cancer in this study which is in line with previous studies (14, 21, 22).
Recent evidence has shown the value of the number of metastatic LNs and LNR to predict postoperative survival (23). Some studies have found a significant relationship between the number of metastatic lymph nodes and survival rate (17). However, the number of lymph nodes involved is more dependent on the number of lymph nodes resected and examined so that this number could increase as the number of resected and examined lymph nodes increase (15, 24, 25). In some studies, the value of the lymph nodes involved is accepted as a weak predictor of survival only when the total number of lymph nodes examined is more than 10; and some studies have stated a median of 7 to 17 to be suitable as the total number of examined lymph nodes (13, 19). In contrast, some studies did not show any significant prognostic value for the number of examined lymph nodes in patients (26, 27).
Using lymph node ratio can control these challenges and provide a better indicator of the total number of lymph nodes and lymph nodes involved (13). Therefore, our focus has been on determining the predictive value of lymph node ratio for patient survival. This study showed LNR can be used as a prognostic factor for survival in patients undergoing Whipple procedure, which is consistent with previous studies (18, 19, 21, 23, 28, 29). LNR was first proposed in 2004 as a predictor for survival in patients with pancreatic cancer in a study evaluating the survival rate in 128 patients undergoing pancreatectomy (13). Subsequently, in a second study on data obtained from 4005 patients with pancreatic cancer (SEER database from 1998 to 2003), LNR was reported as a prognostic factor for survival in patients (30). If this ratio is zero in patients, the highest survival rate of 1, 3, and 5 years would be expected (15, 31). Subsequent reports suggested that if this ratio was greater than zero, the mortality rate may even follow a linear pattern and increase with the increased LNR (14). However, researchers tend to categorize this ratio and determine a cut-off for it (19).
In the present study, the greatest difference in survival rate was seen at a cut-off point of 0.20, so that patients with 0 < LNR ≤ 0.2 had a higher 1, 3, and 5-year survival rate compared with those with an LNR > 0.2 and the lowest 1-year (62%) and three-year (25%) survival rate was seen in patients with LNR > 0.2 (although in this cohort no patient had an LNR greater than 0.20 and followed for up to 5 years). In general, determining the appropriate cut-off point is challenging when using any continuous quantitative ratio, because the cut-off must be able to separate statistically significant levels. In some previous studies, the median of this ratio was used as the cut-off point (25), but in the present study, we considered the median and the cut-off most studies reported as suitable (21).
We evaluated the survival rate of patients at the 2 cut-off points of 0.20 and 0.17. House et al. evaluated data from 696 patients with pancreatic cancer and found the highest difference in survival rate when a cut-off point of 0.18 was considered and patients with LNR > 0.18 had the weakest survival rate (28). Berger et al. also selected a cut-point of 0.15 in evaluating the data of 124 patients (13). In general, researchers have selected cut-off points of 0.15 to 0.2. Many previous studies on patients with gastrointestinal malignancies including pancreatic cancer have reported a significant association between lymph node status and patient survival, and lymph node involvement has been suggested as a prognostic factor for poor survival (13, 14, 17, 19, 29, 31). The results of this study also showed that lymph node involvement could be a prognostic factor for pancreatic cancer in patients with metastatic lymph node (N1), in whom lower 1, 3, and 5-year survival rates were seen compared to patients without lymph node involvement (N0).
The major limitation of the study was the sample size. Although the cut-off point of 0.2 would predict patient survival, it should be taken into consideration that 60 patients were N0 and few patients (16 cases) had LNR ≥ 0.2. As well, the small sample size in each different stage may affect the survival distinction between the groups and so we did not find any significant relationship between cancer stage and survival rate. Therefore, the results of this study should be confirmed by subsequent larger or multicenter studies. Another limitation was few examined lymph nodes in some cases which is related to the suboptimal examination of lymph nodes.
5.1. Conclusion
LNR is a valuable indicator that can be used in patients with lymph node involvement as a prognostic factor for poor survival after the Whipple procedure. The lowest 1, 3, and 5-year survival rates were seen in patients with LNR > 0.20.